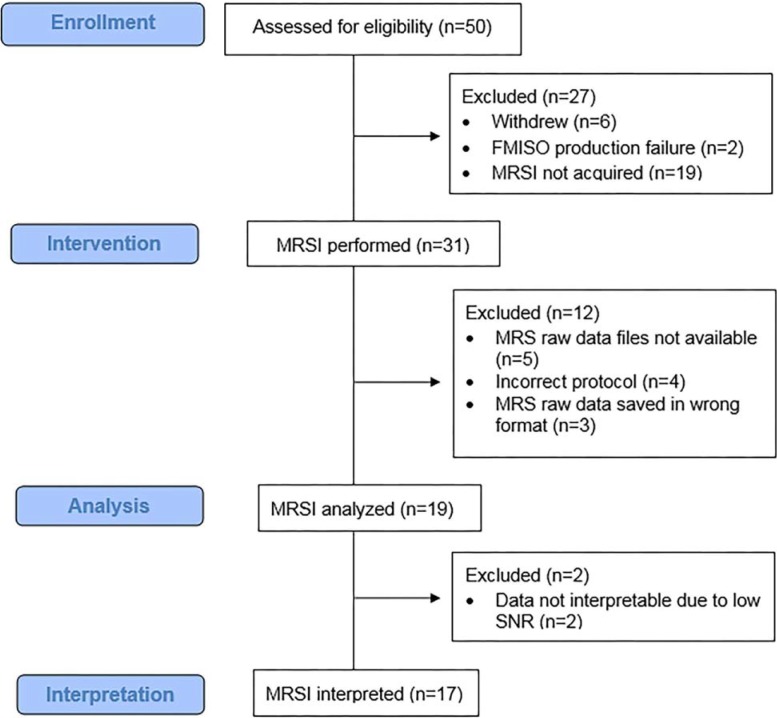
Fig 1. Consort flowchart.
The ACRIN 6684 trial enrolled 50 participants, 27 participants were excluded due to withdrawal (n = 6), technical difficulty in 18F-FMISO production (n = 2) and because MRSI was not performed (n = 19). MRSI was attempted in 31 cases. 17 (55%) had evaluable MRSI data. Reasons for excluding data included: 1. MRS raw data had not been saved at the time of data acquisition (N = 5), 2. Incorrect protocol had been chosen (N = 4), 3. MRS raw data had not been saved in the right format (N = 3), and 4. Data were not interpretable due to low SNR (N = 2).