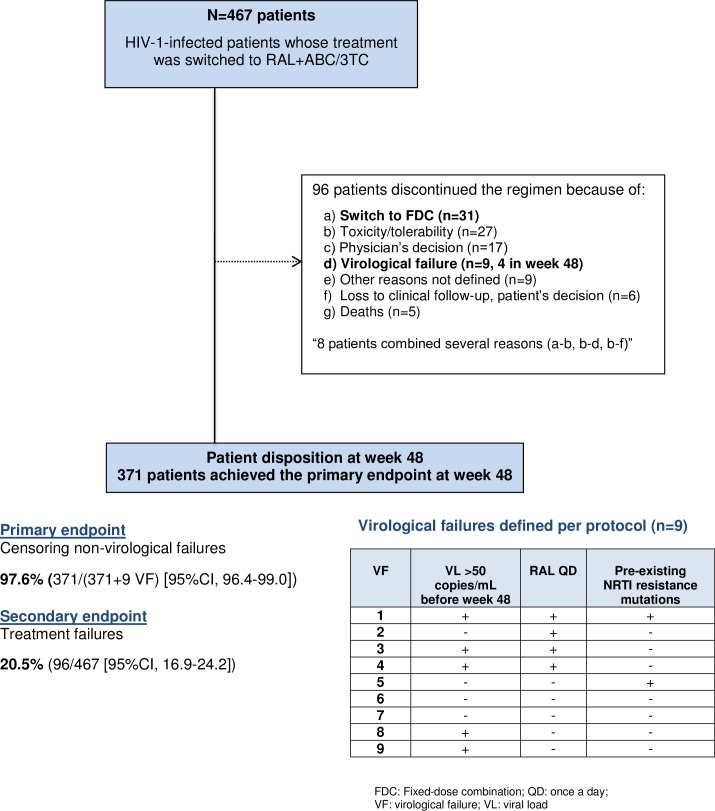
Fig 1. Study flowchart.
Of the 9 patients who experienced protocol-defined virological failure, resistance testing was performed in 7 of the 8 patients with a viral load of more than 1,000 copies/m. Mutations compromising RAL, ABC, or 3TC were detected in 6 patients (1.3%). All 3 drugs were involved in 4 patients (0.8%). The mutations included NRTI-resistance mutations (K65R, K70T, L74V, M184V, and T215F) and INSTI resistance mutations (N155H, L163E, and G163H). Previous genotyping showed that 2 of these patients harbored pre-existing NRTI resistance mutations (K65R, K70Q/T, L74I/V, and M184V) before switching to RAL+ABC/3TC. Four of the patients with protocol-defined virological failure were taking RAL once daily following the off-label dosage indication [11], with pre-existing NRTI resistance mutations in 1 of them.