Figure 4. The DIV pore loop of the human Nav1.7 homology model interacts with β1.
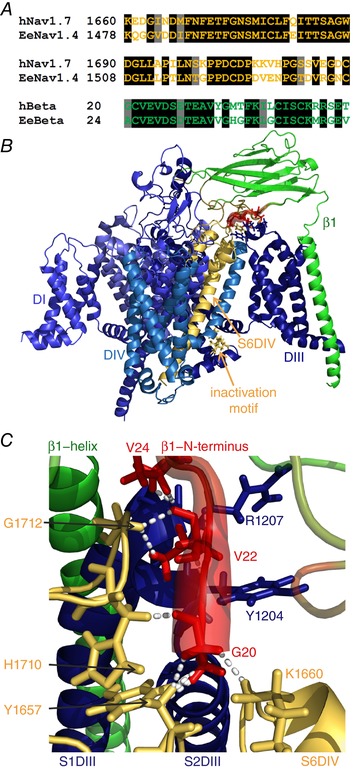
A, sequence alignment of the human Nav1.7 and the electric eel Nav1.4 in the pore loop (first two lines), and of the electric eel and human β1. The sequence identities for the shown parts are 72.8% for the pore loop and 66.6% for β1 (black background: identical residues; grey background: similar residues). B, overview of the homology model of the human Nav1.7 with the human β1 subunit. The colouring is identical to that in Fig. 3, with simulated electric eel β1 RMSF values being mapped onto the human β1 model. The overall structure resembles the electric eel structure. C, close‐up of β1 interface in contact with hNav1.7. Colouring and diameters are the same as in Fig. 3 to illustrate the flexible parts of β1 in red. Dashed lines represent interactions between the first 10 N‐terminal amino acids of β1 and the α subunit with a cut‐off defined at 2.3 Å. Gly20 of β1 interacts with Tyr1657 and His1710 on the S5–S6DIV, and Lys1660 on S6DIV; Val22 of β1 is in contact with Tyr1204 and Arg1207 on S1–S2DIII, and Gly1712 on the S5–S6DIV. Val24 of β1 additionally contacts the backbone of Arg1207 on S1–S2DIII. Interacting residues are displayed in stick representation. The human model of the Nav1.7–β1 interface strongly resembles the electric eel model. This is especially the case for the interaction between the N‐terminus and the pore loop (S5–S6DIV).
