Figure 1.
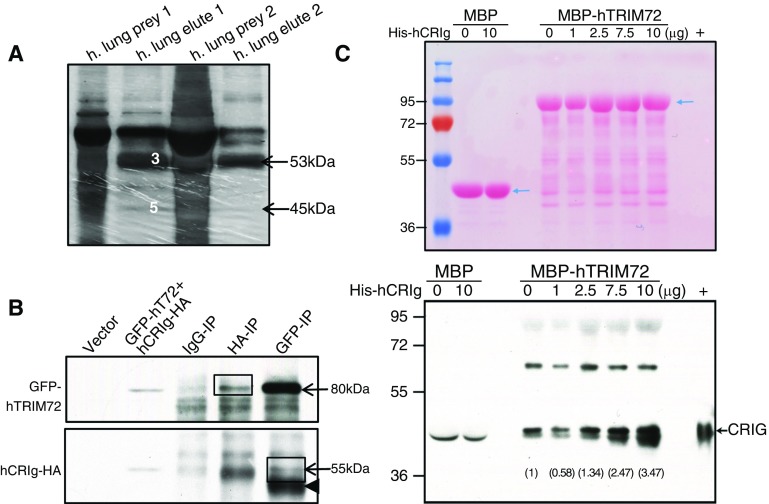
Complement receptor (CR) of the Ig superfamily (CRIg) is a novel interacting partner of tripartite motif protein (TRIM) 72 in the lung. (A) Silver staining of prey (human lung lysates) and elutes in two in-column poly-his–tagged protein interaction assays. Target proteins were identified by mass spectrometry, including human TRIM (hTRIM) 72 (band 3 in prey1 and arrow in prey2, ∼53 kD) and human CRIg (hCRIg; short form, band 5 in prey1 and arrow in prey2, ∼45 kD). (B) Coimmunoprecipitation of GFP-tagged hTRIM72 (GFP-hT72) and hemagglutinin-tagged hCRIg (hCRIg-HA) in human embryonic kidney (HEK) 293 cells; arrows, GFP-T72 at roughly 80 kD, hCRIg-HA at roughly 55 kD (long form); arrowhead, IgG heavy chain; IP = immunoprecipitation; box, specific interaction bands. (C) Ponceau S staining (upper panel) and Western blot detection of CRIg (lower panel) in the gradient binding elutes using approximately 0.4 μM recombinant maltose binding protein (MBP) or MBP-hTRIM72 with a designated amount of His-hCRIg protein (short form, ∼45 kD); arrows, MBP or MBP-hTRIM72 (upper panel), CRIg (lower panel); +, His-hCRIg. Note: a doublet was detected by anti-CRIg close to the “+” control, but only the upper band is a specific CRIg band. Gray values (numbers) of the CRIg band were quantified and labeled on the Western image. Experiments were repeated three times.