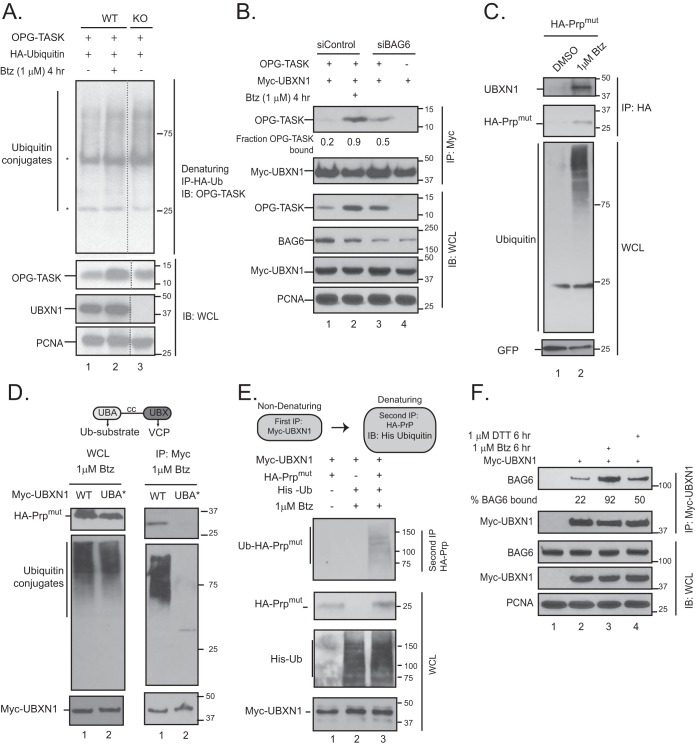
FIG 6.
UBXN1 recognizes ubiquitylated substrates in a BAG6-dependent manner. (A) Wild-type and UBXN1 KO cells were transfected with OPG-TASK and HA-ubiquitin (Ub). Cells were denatured in 2% SDS lysis buffer, and HA-ubiquitin was affinity purified and probed for ubiquitylated OPG-TASK (*, heavy and light chains). (B) HEK-293T cells were transfected with BAG6 siRNA, OPG-TASK, and Myc-UBXN1. Myc-UBXN1 immunoprecipitates were probed for OPG-TASK. The fraction of OPG-TASK bound to UBXN1 was calculated by densitometry and normalized to the levels of OPG-TASK in the cell lysate. (C) HA-PrPmut was transiently expressed in HEK-293T cells that were treated with 1 μM bortezomib for 2 h. Stabilized HA-PrPmut was immunoprecipitated via the HA tag and probed for UBXN1. GFP was used as a transfection control. (D) Domains within UBXN1. UBA, ubiquitin associated; cc, coiled coil; UBX, ubiquitin X. HEK-293T cells were transfected with HA-PrPmut and wild-type Myc-UBXN1 or the UBA M13A F15A mutant (UBA*). Cells were treated with bortezomib for 2 h, and Myc-UBXN1 was immunopurified. (E) HeLa Flp-in T-REX cells were transfected with HA-PrPmut, Myc-UBXN1, and His-ubiquitin and treated with 1 μM bortezomib, as indicated. Consecutive nondenaturing (for Myc-UBXN1) and denaturing (for HA-PrPmut) immunoprecipitations were performed, and immunoprecipitates were probed for His-ubiquitin to demonstrate that PrP bound to UBXN1 is ubiquitylated. (F) HEK-293T cells were transfected with Myc-UBXN1 and treated with 1 μM bortezomib or 1.5 μM DTT for 6 h. Myc-UBXN1 was purified from cell lysates and probed for BAG6. The levels of BAG6 bound to UBXN1 were determined by densitometry and normalized to BAG6 levels in the whole-cell lysate (n = 3).