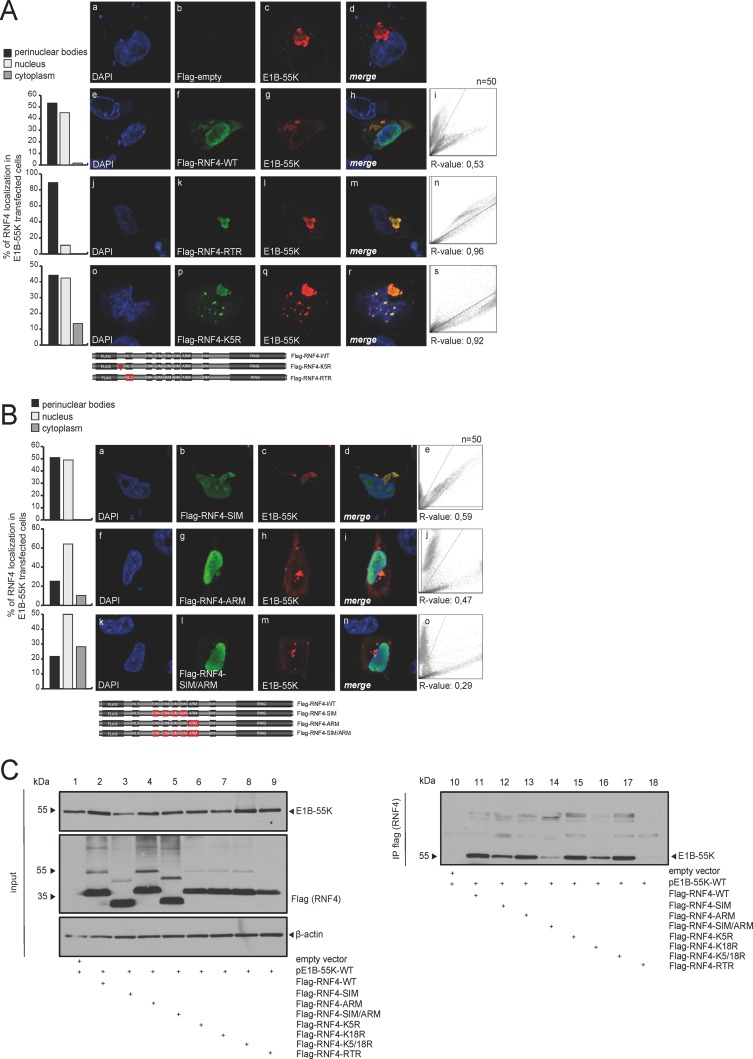
FIG 3.
E1B-55K binding is mediated by several regions in the RNF4 protein. (A) H1299 cells were cotransfected with 2 μg of pE1B-55K and 2 μg pFlag-empty, pFlag-RNF4-WT, RTR, or K5R. Cells were fixed with 4% PFA after 48 h posttransfection and labeled with α-Flag mouse MAb M2 (Sigma-Aldrich, Inc.), detected with Alexa 488 (α-Flag; green)-conjugated secondary antibody. Representative α-Flag (green; Bb, Bf, Bk, Bp), α-E1B-55K (red; Bc, Bg, Bl, Bq), and DAPI (blue; Ba, Be, Bj, Bo) staining patterns, overlays of the single images (merge; Bd, Bh, Bm, Br), and two-dimensional intensity histograms (Bi, Bn, Bs) are shown (n = 50 cells). Schematic representation of pFlag-RNF4-WT, the pFlag-RNF4-RTR (3-amino acid [aa] mutation in the putative NLS signal K192021R), and pFlag-RNF4-K5R construct (1-aa mutation in the putative ubiquitinylation site). Mutated regions were marked in red. (B) H1299 cells were cotransfected with 2 μg of pE1B-55K and 2 μg pFlag-RNF4-SIM, ARM, or SIM/ARM. Cells were fixed with 4% PFA after 48 h posttransfection and labeled as indicated in panel A. Representative α-Flag (green; Cb, Cg, Cl), α-E1B-55K (red; Cc, Ch, Cm), and DAPI (blue; Ca, Cf, Ck) staining patterns, overlays of the single images (merge; Cd, Ci, Cn), and 2D intensity histograms (Ce, Cj, Co) are shown (n = 50 cells). Schematic representation of the mutated pFlag-RNF4 constructs SIM (deletion of SIM1-4), ARM (deletion of ARM, positions 73 to 83), and SIM/ARM (deletion of SIM1-4 and ARM). Mutated regions were marked in red. Colocalization of Flag-RNF4 and E1B-55K was analyzed using coloc2 in Fiji (30) and calculated using Pearson's correlation coefficient (R value). (C) H1299 cells were cotransfected with a plasmid encoding E1B-55K and pFlag-RNF4-WT, SIM, ARM, SIM/ARM, K5R, K18R, K5/18R, and RTR and harvested 48 h posttransfection, and total cell extracts were prepared. Immunoprecipitation of pFlag-RNF4 was performed using α-Flag mouse MAb M2 (Sigma-Aldrich, Inc.). Proteins were separated by SDS-PAGE and subjected to immunoblotting. Input levels of total cell lysates and coprecipitated proteins were detected using mouse MAb 2A6 (α-E1B-55K), anti-Flag mouse MAb M2 (Sigma-Aldrich, Inc.), and mouse MAb AC-15 (α-β-actin) as a loading control. Molecular sizes, in kDa, are indicated on the left, and relevant proteins are on the right.