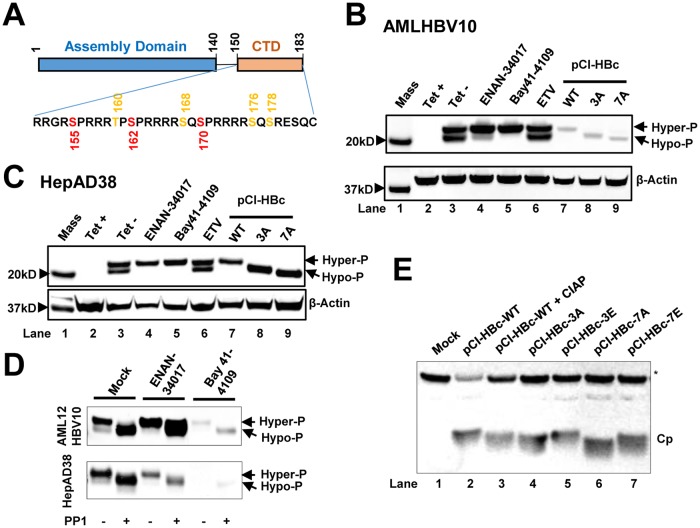
FIG 1.
HBV capsid assembly modulators alter the phosphorylation status of HBV core protein. (A) Schematic representation of the domain structure of HBV core protein. The three major (red) and four minor (orange) phosphor acceptor sites are indicated. (B and C) AML12HBV10 (B) or HepAD38 (C) cells cultured in the absence of Tet were mock treated or treated with 2 μM Bay 41-4109, 5 μM ENAN-34017, or 0.1 μM ETV for 2 and 6 days, respectively. HepG2 cells were transfected with plasmid pCI-HBc-WT (WT), pCI-HBc-3A (3A), or pCI-HBc-7A (7A) and harvested at 72 h posttransfection. Intracellular core proteins were analyzed by a Western blot assay with antibody HBc-170A. β-Actin served as a loading control. (D) AML12HBV10 and HepAD38 cells were mock treated or treated with 2 μM Bay 41-4109 or 5 μM ENAN-34017 for 2 or 8 days, respectively. Intracellular HBV capsids were pelleted by ultracentrifugation and mock treated or treated with protein phosphatase 1 for 16 h at 30°C. HBV core proteins were detected by a Western blot assay with HBc-170A antibody. (E) Wild-type (WT) and mutant core proteins (Cp) with three major or all seven phosphoacceptor sites replaced with A or E were synthesized in RRL, with or without CIAP treatment, and analyzed by a Western blot assay with antibody HBc-170A. The nonspecific band labeled with an asterisk served as a loading control. Hyper-P and Hypo-P, hyper- and hypophosphorylated core protein, respectively.