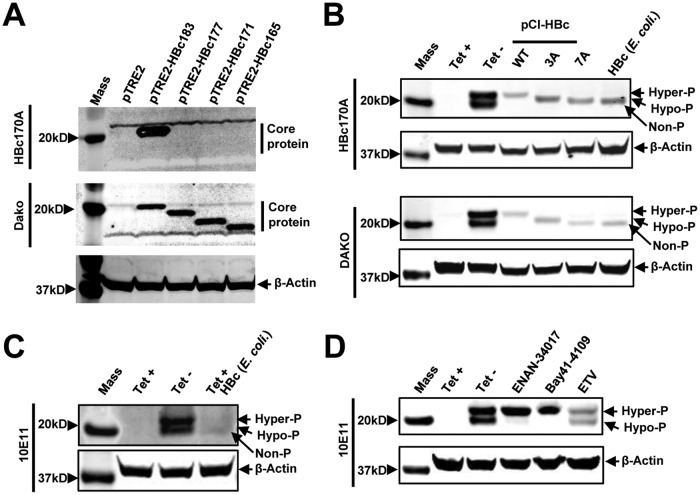
FIG 2.
Characterization of core protein phosphorylation status with three antibodies against HBV core proteins. (A) AML12 cells were cotransfected with pTet-off and vector plasmid (pTRE2) or a plasmid expressing either wild-type full-length core protein (pTRE2-HBc183) or the indicated C-terminally truncated core proteins. The cells were harvested at 48 h posttransfection. (Top and middle) Core protein expression was detected by Western blotting assay with antibody HBc170A (top) or anti-core antibody from Dako (middle). (Bottom) β-Actin served as a loading control. (B) Cell lysates from AML12HBV10 cells cultured in the presence or absence of Tet for 2 days and HepG2 cells transfected with plasmid pCI-HBc-WT, pCI-HBc-3A, or pCI-HBc-7A and harvested at 72 h posttransfection, as well as HepG2 cell lysate containing 10 ng HBV core protein expressed in E. coli, were resolved by SDS-PAGE. HBV core protein was detected with antibody HBc170A or Dako antibody. β-Actin served as a loading control. (C) Cell lysates from AML12HBV10 cells cultured in the presence or absence of Tet for 2 days and cell lysates of AML12HBV10 cells (cultured in the presence of Tet) containing 10 ng HBV core protein expressed in E. coli were detected by a Western blot assay with antibody 10E11. β-Actin served as a loading control. (D) AML12HBV10 cells were mock treated or treated with 2 μM Bay 41-4109, 5 μM ENAN-34017, or 0.1 μM ETV for 2 days. Intracellular core proteins were analyzed by a Western blot assay with antibody 10E11. β-Actin served as a loading control. Non-P, nonphosphorylated core protein.