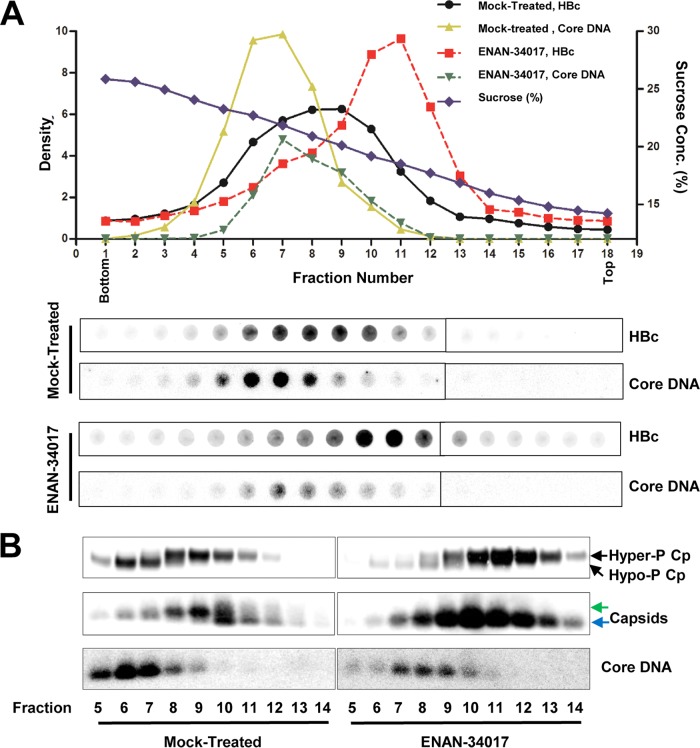
FIG 3.
HBV core protein is hyperphosphorylated in empty capsids but hypophosphorylated in DNA-containing capsids. (A) AML12HBV10 cells were cultured in Tet-free medium and left untreated or treated with 5 μM ENAN-34017 for 2 days. Intracellular capsids were sedimented on a sucrose gradient (15 to 30%) with a Beckman SW28 rotor. Eighteen equal-volume (1.5-ml) fractions were collected from the bottom of the tube, and. 50 μl of each fraction was applied to a nylon membrane. The membrane was probed with an antibody against HBc (Dako) to detect HBc and then hybridized with a minus-strand-specific full-length HBV riboprobe to detect HBV DNA. Relative amounts of HBV core protein and DNA were quantified with a Li-Cor Odyssey system and a Typhoon phosphorimager system, respectively. The dot blot images for each of the assays were derived from two consecutive rows of a 96-well dot blot manifold. (B) HBV capsids in the remaining samples of fractions 5 to 14 (highlighted by the red box in panel A) were pelleted by ultracentrifugation and dissolved in TNE buffer. One half of the sample from each fraction was used for Western blot analysis of core protein with antibody HBc-170A and the other half for analyses of capsids and their associated HBV DNA in 1.5% native agarose gel assays. The slow- and fast-migrating capsids are indicated by green and blue arrows, respectively.