FIG 7.
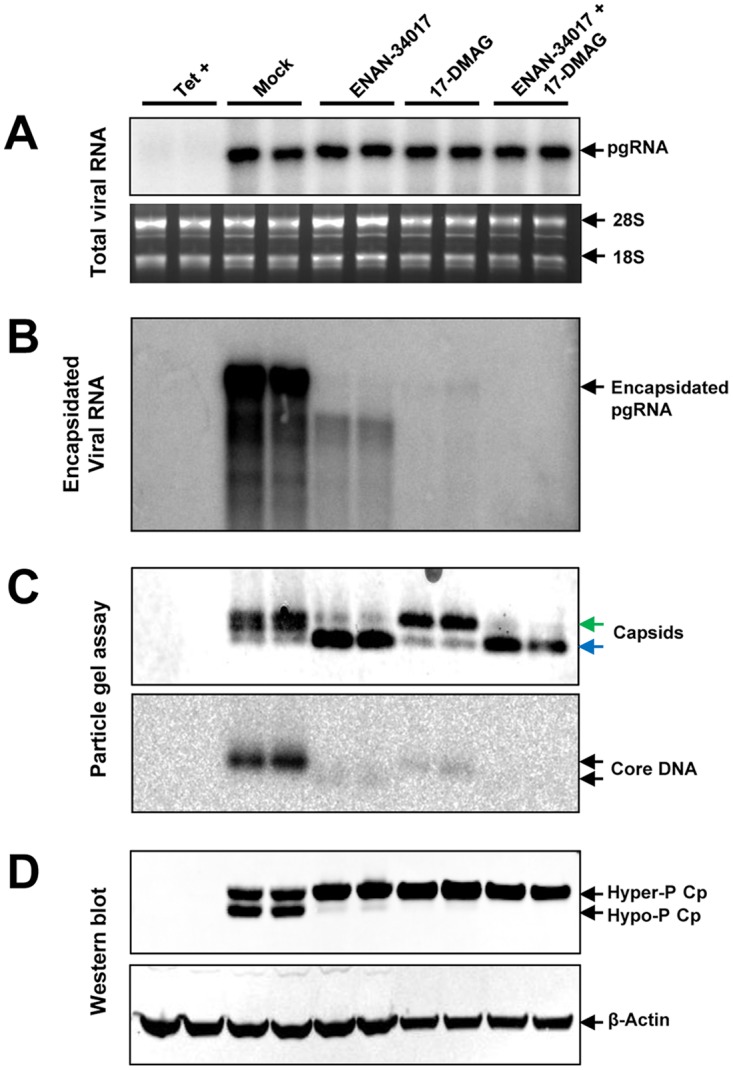
Disruption of pgRNA encapsidation by an HSP90 inhibitor reduced the amount of dephosphorylated core protein. AML12HBV10 cells were mock treated or treated with 5 μM ENAN-34017 or 300 nM 17-DMAG, alone or in combination, in the absence of Tet for 2 days. (A) Total and encapsidated pgRNAs were determined by Northern blotting hybridization assays. 28S and 18S rRNAs served as loading controls. (B) Encapsidated pgRNA was determined by Northern blotting hybridization assay. (C) Cytoplasmic HBV capsids were analyzed by a particle gel assay with 1.8% agarose gel electrophoresis. The slow- and fast-migrating capsids are indicated by green and blue arrows, respectively. Capsid-associated HBV DNA was determined by hybridization with a full-length riboprobe specific for minus-strand HBV DNA. (D) The phosphorylation status of core protein was determined by a Western blot assay with antibody HBc-170A. β-Actin served as a loading control.
