Fig. 5.1.
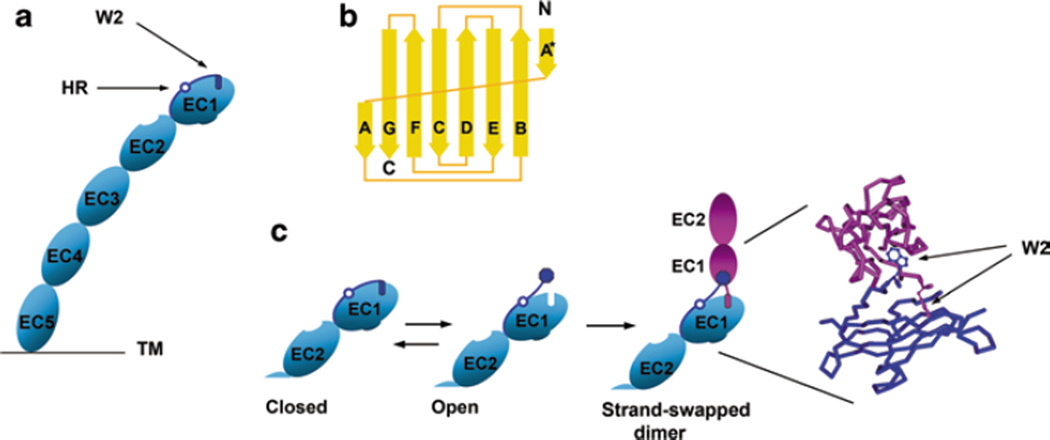
Cadherin dimerization using the strand-swap interface. a Schematic representation of the cadherin ectodomain. It consists of five homological cadherin-like domains (EC1–EC5). The A* and A strands of EC1 are dark blue. They are separated by the hinge region (open circle, HR). The most important residue of the A* strand is Trp2 (dark blue rectangle, W2). The cadherin molecule shown is in the closed conformation—its Trp2 residue is inserted into its own core. b Topology diagram of the classical cadherin EC1 domain. Note that the domain consists of seven β strands. The first strand is broken into two parts, strands A* and A. Strand A* forms a contact with strand B. This interaction can be intra- or inter-molecular. c Schematic representation of the strand-swapping process. Only EC1 and EC2 domains are shown. In the presence of calcium ions, the closed cadherin conformation is unstable and is in equilibrium with the open conformation in which Trp2 is exposed to solvent. Two cadherin molecules in open conformation produce a strand-swap cadherin dimer. The structural model of the strand-swapped dimer (only EC1 domains of both molecules are shown) is on the right. Note that the W2 residues of both molecules in the dimer are in nearly perpendicular planes