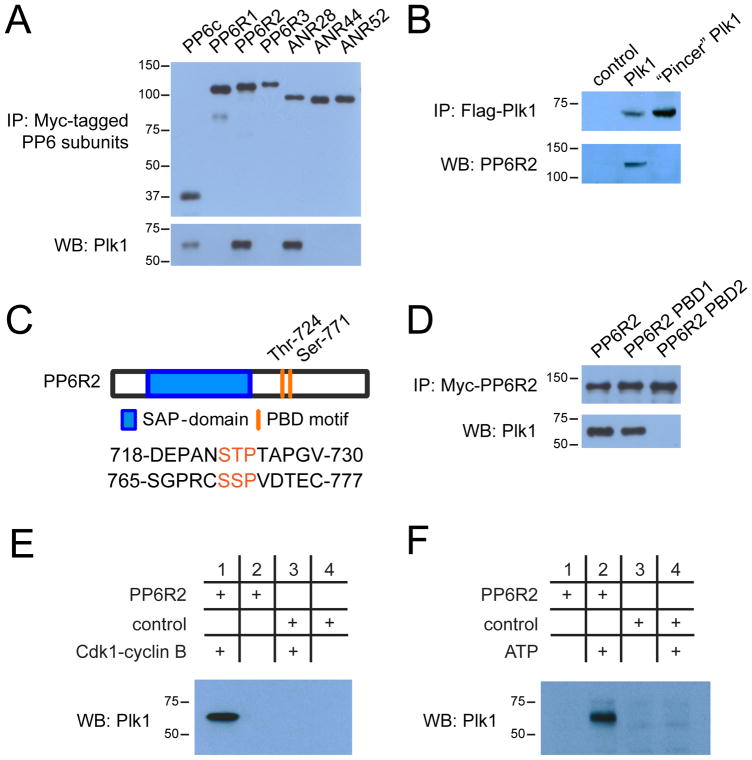
Figure 4. Regulatory interaction of PP6 and Plk1.
(A) Western blot analysis of Myc-tagged PP6 subunit binding to endogenous Plk1 in immunoprecipitates (IP) from lysates of HeLa cells expressing the indicated Myc-tagged PP6 subunit. (B) Immunoprecipitation of Flag-tagged wild-type and “Pincer” mutant Plk1 from transfected HeLa cells and Western blot analysis of co-precipitated endogenous PP6R2. (C) Diagram depicting PP6R2 domain structure and location of PBD motifs. (D) Immunoprecipitations of wild-type, PBD1-, and PBD2-mutant PP6R2 and Western blot analysis of co-precipitated Plk1. (E) Immunoprecipitation assessing the dependence of the PP6R2-Plk1 interaction on the activity of CDK1-cyclin B complex. Bacterially expressed and purified PP6R2 was immobilized on activated Sepharose and incubated with ATP and CDK1-cyclin B complex or not, followed by addition to mitotically-arrested (Taxol) HeLa cell lysate, isolation of PP6R2-containing complexes and Western blotting for Plk1. (F) As described in (E), except that immobilized PP6R2 was incubated directly with mitotic HeLa cell lysates with or without additional ATP prior to isolation of PP6R2 complexes and Western blotting for Plk1. BSA-Sepharose was used as a control (E and F).