Figure 5.
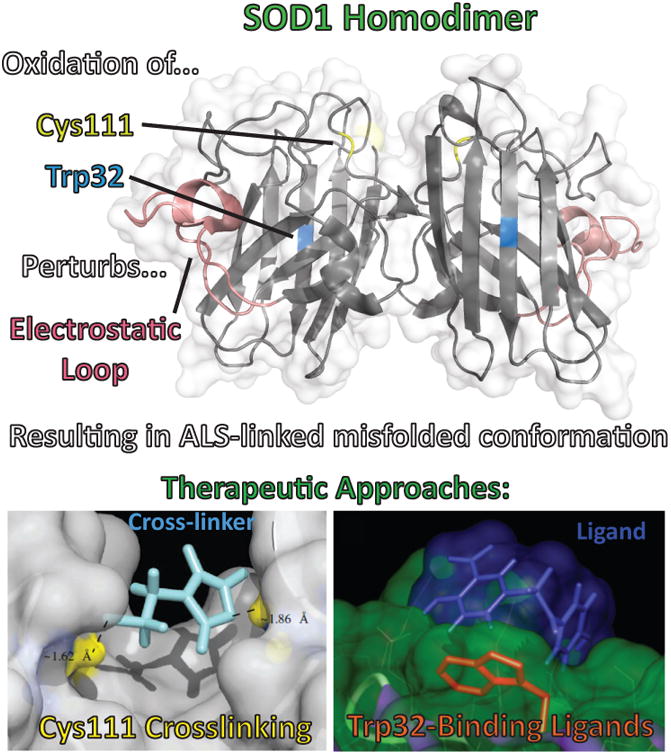
(Top) Oxidation of SOD1's Cys111 and Trp32 perturb the structure of the protein in a similar way as any of the pathogenic disease variants, leading to ALS-linked misfolded conformations of the protein. (Bottom) Therapeutic approaches that have been proposed to prevent these oxidation events are (Left) cross-linking proximal Cys111's on SOD1 monomer pairs to block the cysteines from oxidation and maintain the homodimer and (Right) blocking oxidation of Trp32 with a ligand, which also serves to stabilize the protein structure.
