Abstract
Objective
Pediatric loss of control (LOC) eating prospectively predicts the worsening of metabolic syndrome components. However, it is unknown if remission of LOC eating is associated with improvements in metabolic health. Therefore, we conducted a secondary analysis of a trial that enrolled adolescent girls with LOC eating, examining whether LOC remission (vs. persistence) at end-of-treatment was associated with changes in metabolic syndrome components at 6-month follow-up.
Method
One hundred three adolescent girls (age 14.5±1.7y; BMI-z 1.5±0.3; 56.3% non-Hispanic White, 24.3% non-Hispanic Black) with elevated weight (75th-97th BMI %ile) and reported LOC eating were assessed for metabolic syndrome components at baseline and again six months following the interventions. The main effects of LOC status at end-of-treatment (persistence vs. remission) on metabolic syndrome components (waist circumference, lipids, glucose, and blood pressure) at 6-month follow-up were examined, adjusting for baseline age, depressive symptoms, LOC frequency, fat mass, and height, as well as race, change in height, change in fat mass, and the baseline value of each respective component.
Results
Youth with LOC remission at end-of-treatment had lower glucose (83.9±6.4 vs. 86.5±5.8 mg/dL; p = .02), higher high-density lipoprotein cholesterol (50.3±11.8 vs. 44.8±11.9 mg/dL; p = .01), and lower triglycerides (84.4±46.2 vs. 96.9±53.7 mg/dL; p = .02) at 6-month follow-up compared to youth with persistent LOC, despite no baseline differences in these components. No other component significantly differed by LOC eating status (ps > .05).
Discussion
Reducing LOC eating in adolescent girls may have a beneficial impact on some components of the metabolic syndrome.
Keywords: loss of control eating, metabolic syndrome, obesity, adolescents, overweight
In concert with the high rates of pediatric obesity, in recent decades there has been a substantial increase during childhood and adolescence in the prevalence of type 2 diabetes (Mayer-Davis et al., 2017) and other aspects of the metabolic syndrome including dyslipidemia and hypertension (Duncan, Li, & Zhou, 2004; Muntner, He, & Cutler, 2004). Moreover, pediatric obesity that persists into adulthood is a risk factor for the development of hypertension, hypercholesterolemia, and type 2 diabetes in adulthood—even if youth do not have metabolic syndrome in childhood (Juonala et al., 2011). Identifying and reducing modifiable factors that promote obesity and its metabolic complications may help prevent the development of full or partial metabolic syndrome (Biltoft & Muir, 2009).
One potentially modifiable risk factor for obesity and metabolic dysfunction is binge eating (American Psychiatric Association, 2013; Hudson et al., 2010). Compared to adults who do not endorse binge eating, adults with binge eating may differ in endocrine and autonomic functioning (Friederich et al., 2006; Lo Sauro, Ravaldi, Cabras, Faravelli, & Ricca, 2008; Messerli-Burgy, Engesser, Lemmenmeier, Steptoe, & Laederach-Hofmann, 2010). Furthermore, the evidence for distinct cardiovascular and psychological phenotypes, even independent of weight status, suggests that binge eating and obesity may be specific phenotypes with respect to psychophysiological outcomes (Klatzkin, Gaffney, Cyrus, Bigus, & Brownley, 2015). Indeed, adults who endorse binge eating episodes are more likely to have hypertension, hypertriglyceridemia, low high-density lipoprotein (HDL-C), insulin resistance, elevated inflammation, and metabolic syndrome than adults without binge eating (Abraham, Massaro, Hoffmann, Yanovski, & Fox, 2014; Succurro et al., 2015). Moreover, binge eating disorder in adults predicts the development of significant health problems, particularly of the cardiovascular and endocrine systems (Hudson et al., 2010), even after accounting for the contributions of weight status.
Loss of control (LOC) eating is a prevalent disordered eating pattern among youth. LOC eating is the subjective experience of an inability to stop eating, and includes eating episodes with an objectively large amount of food (i.e., binge eating) as well as episodes with an ambiguously large amount of food (Tanofsky-Kraff, Marcus, Yanovski, & Yanovski, 2008). For children and adolescents, it appears that the experience of LOC eating, rather than the amount of food consumed, is the most salient indicator of aberrant eating (Shomaker et al., 2010). Approximately 3–5% of youth of healthy weight (Allen, Byrne, La Puma, McLean, & Davis, 2008; Tanofsky-Kraff et al., 2004) and 20–35% of weight loss treatment-seeking youth (Bishop-Gilyard et al., 2011; Glasofer et al., 2007) report recent LOC eating. Compared with adolescents who do not report LOC eating, those with LOC are more likely to be overweight and to have greater adiposity (Morgan et al., 2002; Schluter, Schmidt, Kittel, Tetzlaff, & Hilbert, 2016; Tanofsky-Kraff et al., 2004). Further, even infrequent LOC eating is predictive of excess weight gain in prospective studies (Field et al., 2003; Tanofsky-Kraff, Yanovski, et al., 2009).
Independent of adiposity, LOC eating also appears to put youth at risk for metabolic dysfunction. A cross-sectional analysis found that presence of LOC eating was associated with higher systolic blood pressure and higher low-density lipoprotein cholesterol (LDL-C) compared to youth without LOC eating, even after adjusting for the association of LOC eating with adiposity (Radin et al., 2015). Prospectively, one study that found pediatric binge eating predicted worsening of components of the metabolic syndrome, namely, triglycerides and central adiposity, over a 5-year follow-up (Tanofsky-Kraff et al., 2012). In the same study, baseline LOC overeating was associated with a 5.33 greater odds of meeting criteria for metabolic syndrome 5-years later (Tanofsky-Kraff et al., 2012). Taken together, preliminary data suggests that LOC eating may place youth at risk for metabolic dysfunction.
If the prospective link between LOC eating and worsening metabolic function reflects a causative pathway, then reducing or eliminating this behavior would be expected to promote improvements in metabolic dysfunction. Therefore, we carried out a secondary analysis of a trial that studied adolescent girls with LOC eating prior to and following interventions aimed at reducing excess weight gain. In the primary outcome analyses for this trial, there were no significant differences in how LOC eating or body mass index (BMI) indices changed between a 12-week interpersonal group psychotherapy experimental program and a standard-of-care group health education program (Tanofsky-Kraff et al., 2014). Therefore, we hypothesized that adolescent girls whose LOC eating remitted, regardless of intervention assignment, would evidence greater improvements in metabolic function compared to girls whose LOC eating persisted.
Methods
Participants
This study is a secondary analysis of healthy adolescent girls, 12–17-years-old, recruited for participation in a randomized controlled clinical trial at the Uniformed Services University of the Health Sciences (USUHS) and the National Institutes of Health (NIH) Hatfield Clinical Research Center in Bethesda, Maryland (ClinicalTrials.Gov ID: NCT00680979). Findings from the primary aims, describing group condition differences on mood, LOC eating, and weight, are reported elsewhere (Tanofsky-Kraff et al., 2014). This manuscript is the first presentation of the study’s metabolic data that were collected as secondary outcomes.
As previously described (Tanofsky-Kraff et al., 2014), all participants were deemed at risk for excess weight gain due to a BMI (kg/m2) between the 75th and 97th percentiles (Field, Cook, & Gillman, 2005) and the report of at least one episode of LOC eating in the previous month (Tanofsky-Kraff, Yanovski, et al., 2009). Girls were excluded from the trial if they had a major medical or psychiatric condition other than binge eating disorder, if they were in behavioral weight loss or psychotherapy, or were taking medications known to affect body weight or appetite, including oral contraceptives. Girls were excluded from this secondary analysis if they did not complete their 6-month follow-up visit (n = 10). Adolescents were recruited through the NIH clinical trials website, local area community flyer postings, and direct mailings to homes within a 50-mile radius of Bethesda, Maryland. Recruitment materials were directed to parents who were concerned about their daughter’s body weight and eating behavior. The study was approved by the USUHS and Eunice Kennedy Shriver National Institute of Child Health and Human Development institutional review boards. Parents and daughters provided written consent and assent, respectively.
Procedure
At a baseline visit, following an overnight fast, girls completed physiological and psychological assessments. Participants were then randomized to a 12-week interpersonal group psychotherapy or group health education control program, as previously described (Tanofsky-Kraff et al., 2014). The interpersonal psychotherapy group was modified from existing programs focused on the prevention of depression in adolescents (Young, Mufson, & Davies, 2006) and the treatment of binge eating disorder (Wilfley, MacKenzie, Welch, Ayres, & Weissman, 2000). The health education program was adapted from the HEY-Durham manual (Bravender, 2005). Follow-up assessment of LOC eating took place at the end of the 12-week intervention and metabolic function was re-assessed six months following the initiation of the programs.
Measures
LOC eating
The Eating Disorder Examination Version 14 OD/C.2 (Fairburn & Cooper, 1993) was administered to assess the presence and frequency of LOC eating at baseline and the presence of LOC eating at end-of-treatment. The presence of LOC eating was defined by at least one objective or subjective binge episode in the previous 28 days. The Eating Disorder Examination has shown good inter-rater reliability and discriminant validity in child and adolescent samples (Glasofer et al., 2007; Tanofsky-Kraff et al., 2004), and in the current sample (Tanofsky-Kraff et al., 2014). As all participants reported LOC eating at baseline, girls were categorized as those with LOC eating persistence (i.e., reported at least one LOC eating episode in the past 28 days at end-of-treatment) or LOC eating remission (i.e., did not report LOC eating in the past 28 days at end-of-treatment).
Depressive Symptoms
Depressive symptoms were measured using the Beck Depression Inventory II (Beck, Steer, & Brown, 1996), a self-report questionnaire containing twenty-one items that query about depressive symptoms such as sadness, anhedonia, and irritability. A total score is calculated by summing all items, each scored from 0 to 3, with higher scores representing increased severity of depressive symptoms. Based on the total score, the severity of depressive symptoms is considered minimal (0–13), mild (14–19), moderate (20–28), or severe (29–63). The Beck Depression Inventory has been shown to be valid and reliable in adolescent samples (Ambrosini, Metz, Bianchi, Rabinovich, & Undie, 1991; Beck, Steer, & Garbin, 1988). In this sample, the Beck Depression Inventory had good internal consistency (Cronbach’s α = 0.82).
Anthropometric measurements
Height (cm) was measured in triplicate by calibrated stadiometer. Weight (kg) was obtained in a fasted state with a calibrated digital scale. BMI (kg/m2) was calculated using average height and weight, and then BMI-z score was generated by adjusting for age and sex according to CDC growth standards (Kuczmarski et al., 2002). Body fat mass (kg) was estimated with dual-energy X-ray absorptiometry (DXA), a validated measure of body composition (Ellis, 2000; Rothney, Brychta, Schaefer, Chen, & Skarulis, 2009), using a calibrated Hologic Discovery instrument (Hologic, Inc., Marlborough, MA). Waist circumference (cm) was measured at the iliac crest with a non-elastic tape measure.
Metabolic function
Fasting triglycerides, plasma glucose, and total cholesterol were measured from blood samples using a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN). A Cobas FARA analyzer was used to directly measure high-density lipoprotein cholesterol (HDL-C) using reagents from Sigma chemical (St. Louis, MO). LDL-C was then calculated using the following formula: total cholesterol – HDL-C – (triglycerides/5). Blood pressure was measured using an automated blood pressure monitor (Dynamap, GE Healthcare) at the right brachial artery while participants were seated. Metabolic functioning was examined continuously due to the lack of consensus for clinical cut-offs for metabolic health components in youth (Agudelo et al., 2014; Prodam et al., 2013; Steinberger et al., 2009; Zimmet et al., 2007).
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics 24 (Armonk, NY). All data were screened for outliers and normality. Across all variables at baseline and 6-month follow-up, nineteen values were identified as extreme outliers (defined as more than three standard deviations from the mean) and were recoded to the next lowest or highest value for that variable. LOC eating frequency at baseline was log-transformed to achieve normality. For all participants, baseline differences between youth with LOC eating persistence versus LOC eating remission at end-of-treatment were assessed using independent samples t-tests and chi-square analyses, as appropriate. For participants with valid measures at both baseline and 6-month follow-up, metabolic and anthropometric variables within each group were examined over time using paired samples t-tests.
Given that the randomized controlled trial found no intervention effect of interpersonal psychotherapy versus health education for LOC eating at any follow-up visit (Tanofsky-Kraff et al., 2014), participants were collapsed across intervention assignment for the primary analyses. Missing data varied across metabolic outcomes due to protocol deviations including an inability to draw blood following two sticks, or issues with the samples (e.g., hemolysis) or with laboratory analysis. In order to maximize sample size for each analysis, a series of seven analyses of covariance (ANCOVAs) were conducted to examine the impact of LOC eating remission at end-of-treatment on metabolic syndrome components (waist circumference, triglycerides, LDL-C, HDL-C, plasma glucose, systolic blood pressure, and diastolic blood pressure), with LOC eating status (persistence vs. remission at end-of-treatment) as the independent variable. Participants may have been excluded from each ANCOVA if they were missing the respective outcome data from the 6-month follow-up or if they were missing data for a covariate. The following covariates were included in each ANCOVA: race (coded as non-Hispanic White vs. other), baseline age (y), baseline depressive symptoms, baseline LOC eating frequency, baseline fat mass (kg), baseline height (cm) change in height from baseline to 6-months (cm), change in fat mass from baseline to 6-months (kg), and the baseline value of each respective metabolic syndrome component. If LOC eating status at end-of-treatment was found to be significant in any ANCOVA, follow-up ANCOVAs were conducted including all covariates, LOC eating status at end-of-treatment, intervention assignment, and the interaction between LOC eating status and intervention assignment to confirm there were no main or interactional effects of intervention condition. All tests were two tailed, and differences were considered significant when p-values were < .05.
Results
Participants were 103 adolescent girls between the ages of 12 and 17 years (M = 14.5 years, SD = 1.7). At baseline, the average BMI-z was 1.5 (SD = 0.3) and 56.3% of participants classified themselves as non-Hispanic White. Participants reported an average of 4.5 (SD = 6.02) LOC eating episodes in the past 28 days at baseline. Two participants met criteria for binge eating disorder (American Psychiatric Association, 2013). At end-of-treatment, 58 (56.3%) reported LOC eating persistence, and 45 (43.7%) remitted from LOC eating. At baseline, adolescents with LOC eating persistence at end-of-treatment did not differ from adolescents with LOC eating remission at end-of-treatment with regard to race/ethnicity, age, BMI-z score, fat mass, height, waist circumference, triglycerides, LDL-C, HDL-C, systolic blood pressure, or diastolic blood pressure. However, youth with LOC eating persistence at end-of-treatment had significantly more LOC eating episodes in the past 28 days at baseline (M = 5.6, SD = 5.7) than youth with LOC eating remission at end-of-treatment (M = 2.5, SD = 3.2; p < .001). Youth with LOC eating persistence also had significantly higher depressive symptoms at baseline (M = 12.1, SD = 7.2) than youth with with LOC eating remission (M = 8.6, SD = 5.8, p = .01). Baseline participant characteristics based on LOC eating status at end-of-treatment follow-up are shown in Table 1.
Table 1.
Participant Characteristics at Baseline Based on LOC Eating Status at End-of-Treatment
| LOC Remission (n = 45) |
LOC Persistence (n = 58) |
p | ||
|---|---|---|---|---|
|
|
|
|
||
| Age in years, M (SD) | 14.3 (1.6) | 14.6 (1.8) | .37 | |
| Race, n (%) | .35 | |||
| Non-Hispanic White | 23 (51.1) | 35 (60.3) | ||
| Non-Hispanic Black | 14 (31.1) | 11 (19.0) | ||
| Hispanic | 4 (8.9) | 5 (8.6) | ||
| Other/Unknown | 4 (8.9) | 7 (12.1) | ||
| BMI-z score, M (SD) | 1.6 (0.3) | 1.5 (0.3) | .33 | |
| Fat Mass (kg), M (SD) | 26.6 (6.5) | 26.0 (5.3) | .61 | |
| Height (cm), M (SD) | 162.5 (7.0) | 162.0 (7.4) | .74 | |
| Waist circumference (cm), M (SD) | 84.1 (9.2) | 86.2 (8.1) | .25 | |
| Triglycerides (mg/dL), M (SD) | 87.4 (44.1) | 93.7 (49.6) | .53 | |
| LDL-C (mg/dL), M (SD) | 82.1 (32.5) | 88.9 (23.1) | .29 | |
| HDL-C (mg/dL), M (SD) | 48.8 (10.3) | 46.6 (9.8) | .29 | |
| Plasma glucose (mg/dL), M (SD) | 87.2 (6.3) | 86.6 (7.2) | .69 | |
| Systolic blood pressure (mmHg), M (SD) | 117.5 (8.0) | 116.9 (9.4) | .77 | |
| Diastolic blood pressure (mmHg), M (SD) | 67.0 (6.0) | 65.8 (5.8) | .32 | |
| LOC eating episodes, past 28 days, M (SD) | 2.5 (3.2) | 5.6 (5.7) | <.001* | |
| Depressive symptoms, M (SD) | 8.6 (5.8) | 12.1 (7.2) | .01* | |
Note. Baseline data presented from all participants (n = 103).
Abbreviations: LOC, loss of control; BMI-z, body mass index adjusted for age and sex; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Significant at p < .05.
For girls with LOC eating remission, baseline and 6-month follow-up values did not differ significantly for BMI-z score, fat mass, triglycerides, LDL-C, HDL-C, systolic blood pressure, or diastolic blood pressure. However, girls with LOC eating remission were taller at 6-month follow-up than at baseline (162.6±7.1 vs. 163.6±6.7 cm; p = .001), had a larger waist circumference at 6-month follow-up than at baseline (84.1±9.2 vs. 87.7±10.7 cm; p = .001), and had lower plasma glucose at 6-month follow-up than at baseline (86.8±6.3 vs. 83.8±6.3 mg/dL; p = .02). Girls with LOC eating persistence did not differ significantly between baseline and 6-month follow-up for BMI-z score, waist circumference, triglycerides, LDL-C, plasma glucose, or systolic blood pressure. However, girls with LOC eating persistence had higher fat mass at 6-month follow-up than at baseline (26.0±5.3 vs. 26.8±6.1 kg; p = .04), were taller at 6-month follow-up than at baseline (161.7±7.3 vs. 162.9±7.0 cm; p < .001), had lower HDL-C at 6-month follow-up than at baseline (46.8±10.0 vs. 44.8±11.9 mg/dL; p = .02), and had higher diastolic blood pressure at 6-month follow-up than at baseline (65.8±5.9 vs. 68.2±7.1 mmHg; p = .02).. Anthropometric and metabolic components at baseline and 6-month follow-up within each LOC eating group is shown in Table 2.
Table 2.
Anthropometric and Metabolic Components at Baseline and 6-Month Follow-Up Within Each LOC Eating Group
| Baseline | 6-Month Follow-Up |
p | ||
|---|---|---|---|---|
|
|
|
|
||
| BMI-z score, M (SD) | ||||
| LOC Remission (n = 45) | 1.6 (0.3) | 1.5 (0.3) | .47 | |
| LOC Persistence (n = 58) | 1.5 (0.3) | 1.5 (0.4) | .32 | |
| Fat mass (kg), M (SD) | ||||
| LOC Remission (n = 44) | 26.4 (6.5) | 27.7 (7.1) | .08 | |
| LOC Persistence (n = 58) | 26.0 (5.3) | 26.8 (6.1) | .04* | |
| Height (cm), M (SD) | ||||
| LOC Remission (n = 44) | 162.6 (7.1) | 163.6 (6.7) | .001* | |
| LOC Persistence (n = 58) | 161.7 (7.3) | 162.9 (7.0) | <.001* | |
| Waist circumference (cm), M (SD) | ||||
| LOC Remission (n = 44) | 84.1 (9.2) | 87.7 (10.7) | .001* | |
| LOC Persistence (n = 50) | 86.3 (8.3) | 88.8 (8.8) | .05 | |
| Triglycerides (mg/dL), M (SD) | ||||
| LOC Remission (n = 36) | 87.8 (45.5) | 86.9 (47.9) | .89 | |
| LOC Persistence (n = 44) | 93.0 (51.7) | 96.9 (53.7) | .55 | |
| LDL-C (mg/dL), M (SD) | ||||
| LOC Remission (n = 32) | 82.6 (32.9) | 77.0 (23.4) | .10 | |
| LOC Persistence (n = 40) | 89.6 (23.6) | 86.9 (21.6) | .42 | |
| HDL-C (mg/dL), M (SD) | ||||
| LOC Remission (n = 41) | 49.3 (10.0) | 50.0 (11.8) | .58 | |
| LOC Persistence (n = 50) | 46.8 (10.0) | 44.8 (11.9) | .02* | |
| Plasma glucose (mg/dL), M (SD) | ||||
| LOC Remission (n = 41) | 86.8 (6.3) | 83.8 (6.3) | .02* | |
| LOC Persistence (n = 49) | 86.8 (7.4) | 86.5 (5.8) | .75 | |
| Systolic blood pressure (mmHg), M (SD) | ||||
| LOC Remission (n = 44) | 117.5 (8.0) | 117.9 (9.5) | .76 | |
| LOC Persistence (n = 56) | 116.8 (9.5) | 118.4 (10.3) | .31 | |
| Diastolic blood pressure (mmHg), M (SD) | ||||
| LOC Remission (n = 44) | 67.0 (6.0) | 66.8 (7.7) | .87 | |
| LOC Persistence (n = 56) | 65.8 (5.9) | 68.2 (7.1) | .02* | |
Note. For each variable, data is presented only for participants who had a valid measure at both baseline and 6-month follow-up.
Abbreviations: LOC, loss of control; BMI-z, body mass index adjusted for age and sex; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Significant at p < .05.
When conducting between-groups analyses, after adjusting for covariates, waist circumference at 6-month follow-up did not significantly differ between youth with LOC eating persistence (M = 88.8 cm, SD = 8.8) versus LOC eating remission at end-of-treatment [M = 87.6 cm, SD = 10.8; F(1, 82) = 0.32, η2p = 0.004; p = .57; Supplemental Table S1]. LDL-C at 6-month follow-up did not significantly differ between youth with LOC eating persistence (M = 86.9 mg/dL, SD = 21.6) versus LOC eating remission at end-of-treatment [M = 77.0 mg/dL, SD = 23.8; F(1, 60) = 1.74, η2p = 0.03; p = .19; Supplemental Table S1]. Systolic blood pressure at 6-month follow-up did not significantly differ between youth with LOC eating persistence (M = 118.4 mmHg, SD = 10.3) versus LOC eating remission at end-of-treatment [M = 118.0 mmHg, SD = 9.6; F(1, 88) = 1.61, η2p = 0.02; p = .21; Supplemental Table S1]. Likewise, diastolic blood pressure at 6-month follow-up did not significantly differ between youth with LOC eating persistence (M = 68.2 mmHg, SD = 7.1) versus LOC eating remission at end-of-treatment [M = 67.1 mmHg, SD = 7.4; F(1, 88) = 2.78, η2p = 0.03; p = .10; Supplemental Table S1].
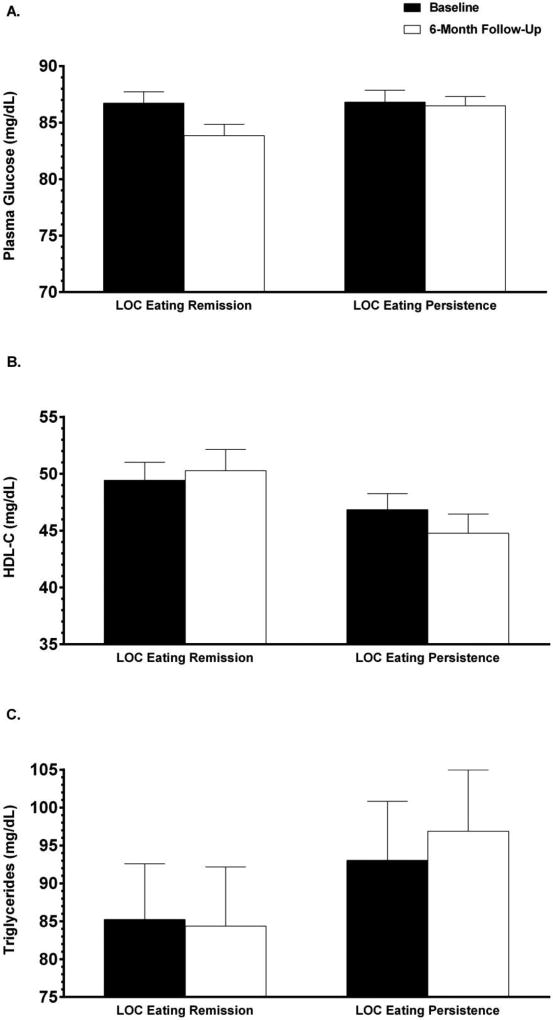
By contrast, after adjusting for covariates, girls with LOC eating persistence had significantly lower HDL-C (M = 44.8 mg/dL, SD = 11.9) than those with LOC eating remission at end-of-treatment [M = 50.3 mg/dL, SD = 11.8; F(1, 79) = 7.26, η2p = 0.08; p = .01; Supplemental Table S1; Figure 1B]. Follow-up analyses confirmed that there were no main (p = .73) effects of intervention condition (interpersonal psychotherapy versus health education) or interaction effects of intervention condition and LOC eating status at 6-month follow-up (p = .95) for HDL-C. Moreover, girls with LOC eating persistence had significantly higher fasting plasma glucose at 6-month follow-up (M = 86.5 mg/dL, SD = 5.8) than those with LOC eating remission [M = 83.9 mg/dL, SD = 6.4; F(1, 78) = 5.76, η2p = 0.07; p = .02; Supplemental Table S1; Figure 1A]. There were no main (p = .54) or interaction effects (p = .87) of intervention condition for plasma glucose. Girls with LOC eating persistence also had significantly higher triglycerides (M = 96.9 mg/dL, SD = 53.7) than those with LOC eating remission at end-of-treatment [M = 84.4 mg/dL, SD = 46.2; F(1, 68) = 5.53, η2p = 0.08; p = .02; Supplemental Table S1; Figure 1C]. No main (p = .44) or interaction effects (p = .82) of intervention condition for triglycerides were identified.
Figure 1.
Unadjusted means and standard errors for metabolic components at baseline and 6-month follow-up are shown by loss of control (LOC) eating status at end-of-treatment. A. Unadjusted means and standard errors for plasma glucose at baseline and 6-month follow-up are shown by LOC eating remission versus persistence at end-of-treatment for participants with data at both time points. After adjusting for covariates including baseline plasma glucose, youth with persistent LOC eating had significantly higher fasting plasma glucose than girls whose LOC eating whose LOC eating had remitted at 6-month follow-up (p = .02). B. Unadjusted means and standard errors for high-density lipoprotein cholesterol (HDL-C) at baseline and 6-month follow-up are shown by LOC eating remission versus persistence at end-of-treatment for participants with data at both time points. After adjusting for covariates including baseline HDL-C, youth with persistent LOC eating had significantly lower HDL-C than girls whose LOC eating whose LOC eating had remitted at 6-month follow-up (p = .01). C. Unadjusted means and standard errors for triglycerides at baseline and 6-month follow-up are shown by LOC eating remission versus persistence at end-of-treatment for participants with data at both time points. After adjusting for covariates including baseline triglycerides, youth with persistent LOC eating had significantly lower triglycerides than girls whose LOC eating had remitted at 6-month follow-up (p = .02).
Discussion
Among adolescent girls at risk for excess weight gain, we observed greater improvements in some metabolic syndrome components at a 6-month follow-up when LOC eating remitted at end-of-treatment. Specifically, adolescents whose LOC eating remitted had lower fasting plasma glucose, higher HDL-C, and lower triglycerides at a 6-month follow-up than adolescents whose LOC eating persisted, despite no baseline differences in these components. These findings complement previous research showing a prospective relationship between LOC eating and the development of metabolic syndrome in youth (Tanofsky-Kraff et al., 2012), and provide preliminary support for the notion that ceasing to engage in LOC eating may improve some metabolic components.
One potential mechanism by which remission of LOC eating could promote improvements in metabolic health is through alteration of macronutrient selection and eating patterns. Despite mixed data on whether youth with LOC eating consume more total energy at meals than their peers without LOC eating, data more consistently demonstrate that intake of youth with LOC eating is distinguished by the consumption of a greater proportion of energy from carbohydrates, including snacks and desserts, and less from protein (Hilbert, Tuschen-Caffier, & Czaja, 2010; Tanofsky-Kraff, McDuffie, et al., 2009; Theim et al., 2007). Dietary patterns that involve a high intake of snacks and desserts have been associated with increased risk of metabolic syndrome in both adults and children (Deshmukh-Taskar et al., 2009; Kelishadi et al., 2008). However, in the current sample, we previously found that compared to the health education control group, snack-type food intake was reduced more in the interpersonal psychotherapy intervention group (Tanofsky-Kraff et al., 2016). While not tested directly, given that we found no main effect of group or interactional effects of group by LOC eating remission on HDL-C, glucose, or triglcyerides, it is likely that the reduction of snack-type food intake does not fully explain the relationship between LOC eating remission and improvements in these metabolic components. Likewise, although our main outcome paper found that interpersonal psychotherapy was more effective at reducing objective binge eating than health education (Tanofsky-Kraff et al., 2014), we found no main effects of group or interaction effects of group by LOC eating remission on these components. Thus, it is also unlikely that a change in objective binge eating episodes would fully explain the relationship between LOC eating remission and improvements in metabolic health. Other features that characterize LOC eating across both subjectively and objectively large episodes may explain the relationship between LOC eating remission and change in metabolic health warrant exploration. For example, increased eating speed has been associated with worsened cardiometabolic health (Lee et al., 2013; Zhu, Haruyama, Muto, & Yamazaki, 2015), and individuals often report eating rapidly during LOC eating episodes (Marcus, Wing, & Hopkins, 1988). Future research should continue to elucidate the relationship between LOC eating, diet quality, and metabolic health as well as examine other potential mechanisms (e.g., depressive symptoms, diet quality, episode size, and other characteristics of LOC eating episodes), between LOC eating and cardiometabolic health.
While previous studies have found that binge eating (LOC with overeating) in adults and LOC eating in youth are associated with increased risk of metabolic syndrome (Abraham et al., 2014; Hudson et al., 2010; Tanofsky-Kraff et al., 2012), not all studies have reported this association (Barber, Schumann, Foran-Tuller, Islam, & Barnes, 2015). When examining associations of specific components of metabolic health, findings have been further mixed. LOC eating in adults has been associated with decreased HDL-C, increased glucose, increased triglycerides, and hypertension (Abraham et al., 2014), while LOC eating in youth has been associated with increased LDL-C and systolic blood pressure (Radin et al., 2015). Prospectively, LOC eating has been linked to dyslipidemia in adults (Hudson et al., 2010) and increased triglycerides in youth (Tanofsky-Kraff et al., 2012). The findings from the present study align with previous studies that have found that LOC eating is associated with dyslipidemia (Abraham et al., 2014; Hudson et al., 2010; Tanofsky-Kraff et al., 2012) and increased glucose (Abraham et al., 2014); however, no association with blood pressure or LDL-C was found in the current study. The lack of consistency in the literature may be due to differences across samples (e.g., severity or duration of LOC/binge eating, age, weight, or sex distribution) or study methodology (e.g., how LOC eating was assessed, covariates included in analyses, or whether outcomes were examined continuously or categorically). It is also possible that LOC eating impacts cardiometabolic health through non-specific pathways, such as inflammation. Cross-sectional analyses have shown that adults (Succurro et al., 2015) and youth (Shank et al., 2017) who report LOC eating have increased markers of inflammation relative to their peers. It is important for future research to examine how LOC eating impacts specific components of metabolic health.
Strengths of the current study include the use of a well-validated, interview measure of LOC eating. The direct estimation of fat mass and metabolic dysfunction using criterion methods are strengths, as opposed to relying on BMI and self-reported metabolic function as in previous studies (Hudson et al., 2010). Yet, longer-term prospective data using larger samples are vital to more fully explore potential mediators and moderators of the relationship between LOC eating and metabolic syndrome. For example, variables that were not measured in this study, such as menstrual cycle phase or duration of LOC eating, may be potential mediators or moderators of the relationship between LOC eating remission and changes in metabolic health. The analysis was also quasi-experimental, as participants were not randomized to LOC eating remission or persistence; therefore, there are several alternative explanations for the observed findings and this analysis should be considered hypothesis generating. Additionally, our sample may lack generalizability, as we recruited only weight gain prevention-seeking, adolescent girls with above average BMI. It is also important to note that this sample was primarily healthy and the changes in metabolic health within each group were not large. However, metabolic components were examined only at 6-month follow-up. It is possible that the effects of LOC eating are cumulative, leading to more clinically significant differences over time. For example, a previous study found that youth with objectively large LOC eating episodes had a 5.33 greater odds of developing metabolic syndrome over the course of five years (Tanofsky-Kraff et al., 2012). Although there is no consensus for clinical cut-offs for components of metabolic health in youth (e.g., Agudelo et al., 2014; Prodam et al., 2013; Steinberger et al., 2009; Zimmet et al., 2007), previous research in young adults suggests that even relatively small increases within the clinically healthy range in metabolic components such as fasting plasma glucose confers increased risk for the development of type 2 diabetes (Tirosh et al., 2005)..
In conclusion, while previous research has found cross-sectional and prospective associations between the presence of LOC eating and metabolic syndrome components, this study has extended this line of research to show that the remission of LOC eating is associated with an improvement in some metabolic syndrome components. Future research should continue to elucidate the relationship between LOC eating and physical health to determine whether remission from LOC eating may improve metabolic health in the long-term. If the remission of LOC eating improves long-term metabolic health, then it may represent a modifiable lifestyle factor that can be targeted to help prevent the development of full or partial metabolic syndrome.
Supplementary Material
Acknowledgments
Funding Sources: NIDDK 1R01DK080906 (to MTK), USUHS grant R072IC (to MTK) and NICHD Intramural Research Program ZIA-HO-00641 (to JAY). No funding sources had any role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Abbreviations
- LOC
loss of control
- NIH
National Institutes of Health
- USUHS
Uniformed Services University of the Health Sciences;
Footnotes
Disclaimers: JAY is a Commissioned Officer of the United States Public Health Service (USPHS). The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the USPHS, the Department of the Navy, USUHS or the U.S. Department of Defense.
Trial Registration: ClinicalTrials.gov ID#: NCT00680979
References
- Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring) 2014;22(11):2441–2449. doi: 10.1002/oby.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo GM, Bedoya G, Estrada A, Patino FA, Munoz AM, Velasquez CM. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: which definition should be chosen for this age group? Metab Syndr Relat Disord. 2014;12(4):202–209. doi: 10.1089/met.2013.0127. [DOI] [PubMed] [Google Scholar]
- Allen KL, Byrne SM, La Puma M, McLean N, Davis EA. The onset and course of binge eating in 8- to 13-year-old healthy weight, overweight and obese children. Eating Behaviors. 2008;9(4):438–446. doi: 10.1016/j.eatbeh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ, Metz C, Bianchi MD, Rabinovich H, Undie A. Concurrent Validity and Psychometric Properties of the Beck Depression Inventory in Outpatient Adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(1):51–57. doi: 10.1097/00004583-199101000-00008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Barber JA, Schumann KP, Foran-Tuller KA, Islam LZ, Barnes RD. Medication Use and Metabolic Syndrome Among Overweight/Obese Patients With and Without Binge-Eating Disorder in a Primary Care Sample. Primary Care Companion for CNS Disorders. 2015;17(5) doi: 10.4088/PCC.15m01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G. The Beck Depression Inventory II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric Properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Biltoft CA, Muir A. The metabolic syndrome in children and adolescents: a clinician’s guide. Adolescent Medicine: State of the Art Reviews. 2009;20(1):109–120. ix. [PubMed] [Google Scholar]
- Bishop-Gilyard CT, Berkowitz RI, Wadden TA, Gehrman CA, Cronquist JL, Moore RH. Weight reduction in obese adolescents with and without binge eating. Obesity (Silver Spring) 2011;19(5):982–987. doi: 10.1038/oby.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravender T. Health, education, and youth in Durham: HEY-Durham curricular guide. 2. Durham, NC: Duke University; 2005. [Google Scholar]
- Deshmukh-Taskar PR, O’Neil CE, Nicklas TA, Yang SJ, Liu Y, Gustat J, Berenson GS. Dietary patterns associated with metabolic syndrome, sociodemographic and lifestyle factors in young adults: the Bogalusa Heart Study. Public Health Nutrition. 2009;12(12):2493–2503. doi: 10.1017/S1368980009991261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Li SM, Zhou XH. Prevalence and Trends of a Metabolic Syndrome Phenotype Among U.S. Adolescents, 1999–2000. Diabetes Care. 2004;27(10):2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- Ellis KJ. Human body composition: In vivo methods.. Physiological Reviews. 2000;80:650–680. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, editors. The Eating Disorder Examination (12th ed.) 12. New York: Guilford Press; 1993. [Google Scholar]
- Field AE, Austin SB, Taylor CB, Malspeis S, Rosner B, Rockett HR, Colditz GA. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112(4):900–906. doi: 10.1542/peds.112.4.900. [DOI] [PubMed] [Google Scholar]
- Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13(1):163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich HC, Schild S, Schellberg D, Quenter A, Bode C, Herzog W, Zipfel S. Cardiac parasympathetic regulation in obese women with binge eating disorder. International Journal of Obesity (London) 2006;30(3):534–542. doi: 10.1038/sj.ijo.0803181. [DOI] [PubMed] [Google Scholar]
- Glasofer DR, Tanofsky-Kraff M, Eddy KT, Yanovski SZ, Theim KR, Mirch MC, Yanovski JA. Binge eating in overweight treatment-seeking adolescents. Journal of Pediatric Psychology. 2007;32(1):95–105. doi: 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiskanen TH, Niskanen LK, Hintikka JJ, Koivumaa-Honkanen HT, Honkalampi KM, Haatainen KM, Viinamäki HT. Metabolic syndrome and depression: a cross-sectional analysis. Journal of Clinical Psychiatry. 2006;67(9):1422–1427. doi: 10.4088/jcp.v67n0913. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen-Caffier B, Czaja J. Eating behavior and familial interactions of children with loss of control eating: a laboratory test meal study. The American Journal of Clinical Nutrition. 2010;91:510–518. doi: 10.3945/ajcn.2009.28843. [DOI] [PubMed] [Google Scholar]
- Hudson J, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, Pope HGJ. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge eating disorder. The American Journal of Clinical Nutrition. 2010;91(6):1568–1573. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juonala M, Magnussen CG, Berenson GS, Venn A, Burns T, Sabin MA, Raitakari OT. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. The New England Journal of Medicine. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Gouya MM, Adeli K, Ardalan G, Gheiratmand R, Majdzadeh R, Group CS. Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN Study. Nutrition, Metabolism & Cardiovascular Diseases. 2008;18(7):461–470. doi: 10.1016/j.numecd.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Kinder LS, Carnethon MR, Palaniappan LP, King AC, Fortmann SP. Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosomatic Medicine. 2004;66(3):316–322. doi: 10.1097/01.psy.0000124755.91880.f4. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Gaffney S, Cyrus K, Bigus E, Brownley KA. Binge eating disorder and obesity: preliminary evidence for distinct cardiovascular and psychological phenotypes. Physiology & Behavior. 2015;142:20–27. doi: 10.1016/j.physbeh.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Lee KS, Kim DH, Jang JS, Nam GE, Shin YN, Bok AR, Cho KH. Eating rate is associated with cardiometabolic risk factors in Korean adults. Nutrition, Metabolism & Cardiovascular Diseases. 2013;23(7):635–641. doi: 10.1016/j.numecd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57(3):95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Wing RR, Hopkins J. Obese binge eaters: affect, cognitions, and response to behavioural weight control. Journal of Consulting and Clinical Psychology. 1988;56(3):433–439. doi: 10.1037//0022-006x.56.3.433. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. The New England Journal of Medicine. 2017;376(15):1419–1429. doi: 10.1056/NEJMc1706291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli-Burgy N, Engesser C, Lemmenmeier E, Steptoe A, Laederach-Hofmann K. Cardiovascular stress reactivity and recovery in bulimia nervosa and binge eating disorder. International Journal of Psychophysiology. 2010;78(2):163–168. doi: 10.1016/j.ijpsycho.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Morgan CM, Yanovski SZ, Nguyen TT, McDuffie J, Sebring NG, Jorge MR, Yanovski JA. Loss of control over eating, adiposity, and psychopathology in overweight children. The International Journal of Eating Disorders. 2002;31(4):430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, He J, Cutler JA. Trends in Blood Pressure Among Children and Adolescents. The Journal of the American Medical Association. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- Prodam F, Ricotti R, Genoni G, Parlamento S, Petri A, Balossini C, Bellone S. Comparison of two classifications of metabolic syndrome in the pediatric population and the impact of cholesterol. J Endocrinol Invest. 2013;36(7):466–473. doi: 10.3275/8768. [DOI] [PubMed] [Google Scholar]
- Radin RM, Tanofsky-Kraff M, Shomaker LB, Kelly NR, Pickworth CK, Shank LM, Yanovski JA. Metabolic characteristics of youth with loss of control eating. Eating Behaviors. 2015;19:86–89. doi: 10.1016/j.eatbeh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30(4):872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17(6):1281–1286. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter N, Schmidt R, Kittel R, Tetzlaff A, Hilbert A. Loss of control eating in adolescents from the community. The International Journal of Eating Disorders. 2016;49(4):413–420. doi: 10.1002/eat.22488. [DOI] [PubMed] [Google Scholar]
- Shank LM, Tanofsky-Kraff M, Kelly NR, Schvey NA, Marwitz SE, Mehari RD, Yanovski JA. Pediatric Loss of Control Eating and High-Sensitivity C-Reactive Protein Concentrations. Childhood Obesity. 2017;13(1):1–8. doi: 10.1089/chi.2016.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Elliott C, Wolkoff LE, Columbo KM, Ranzenhofer LM, Yanovski JA. Salience of loss of control for pediatric binge episodes: does size really matter? The International Journal of Eating Disorders. 2010;43(8):707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Metabolism Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119(4):628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, Arturi F. Obese Patients With a Binge Eating Disorder Have an Unfavorable Metabolic and Inflammatory Profile. Medicine (Baltimore) 2015;94(52):e2098. doi: 10.1097/MD.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Crosby RD, Vannucci A, Kozlosky M, Shomaker LB, Brady SM, Yanovski JA. Effect of adapted interpersonal psychotherapy versus health education on mood and eating in the laboratory among adolescent girls with loss of control eating. The International Journal of Eating Disorders. 2016;49(5):490–498. doi: 10.1002/eat.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Marcus MD, Yanovski SZ, Yanovski JA. Loss of control eating disorder in children age 12 years and younger: proposed research criteria. Eating Behaviors. 2008;9:360–365. doi: 10.1016/j.eatbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, Yanovski JA. Laboratory assessment of the food intake of children and adolescents with loss of control eating. The American Journal of Clinical Nutrition. 2009;89(3):738–745. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Yanovski SZ. A prospective study of pediatric loss of control eating and psychological outcomes. Journal of Abnormal Psychology. 2011;120(1):108–118. doi: 10.1037/a0021406. doi:0.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Stern EA, Miller R, Sebring N, Dellavalle D, Yanovski JA. Children’s binge eating and development of metabolic syndrome. International Journal of Obesity. 2012;36(7):956–962. doi: 10.1038/ijo.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Wilfley DE, Young JF, Sbrocco T, Stephens M, Yanovski JA. Targeted prevention of excess weight gain and eating disorders in high-risk adolescent girls: a randomized controlled trial. The American Journal of Clinical Nutrition. 2014;100(4):1010–1018. doi: 10.3945/ajcn.114.092536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. The International Journal of Eating Disorders. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. Journal of Consulting and Clinical Psychology. 2004;72(1):53. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theim KR, Tanofsky-Kraff M, Salaita CG, Haynos AF, Mirch MC, Ranzenhofer LM, Yanovski JA. Children’s descriptions of the foods consumed during loss of control eating episodes. Eating Behaviors. 2007;8(2):258–265. doi: 10.1016/j.eatbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. The New England Journal of Medicine. 2005;353(14):1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, MacKenzie KR, Welch RR, Ayres VE, Weissman MM. Interpersonal psychotherapy for group. New York, NY: Basic Books; 2000. [Google Scholar]
- Young JF, Mufson L, Davies M. Efficacy of Interpersonal Psychotherapy- Adolescent Skills Training: an indicated preventive intervention for depression. Journal of Child Psychology and Psychiatry. 2006;47:1254–1262. doi: 10.1111/j.1469-7610.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- Zhu B, Haruyama Y, Muto T, Yamazaki T. Association between eating speed and metabolic syndrome in a three-year population- based cohort study. Journal of Epidemiology. 2015;25:322–336. doi: 10.2188/jea.JE20140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S IDF Consensus Group. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatric Diabetes. 2007;8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.