Scheme 2.
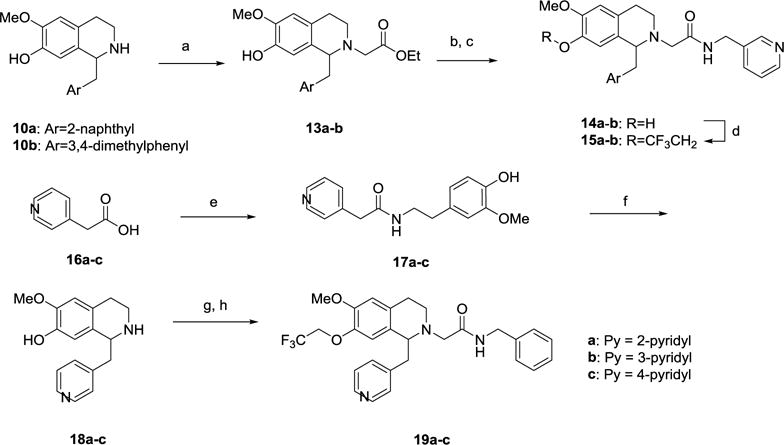
Synthesis of 7-trifluoroethoxy substituted THIQ derivatives 19a-c.a
aReagents and Conditions: (a) BrCH2CO2Et, iPr2EtN, Bu4NI, DMF, rt, 16 h; (b) 2N NaOH (aq), EtOH, rt, 16 h; (c) 3-aminomethylpyridine, HATU, iPr2EtN, DMF, rt, 16 h; (d) CF3CH2I, Cs2CO3, DMF, 100 °C, 2 h; (e) 4-Hydroxy-3-methoxyphenylethylamine, HBTU, iPr2EtN, DMF or DCC, HOBt, Et3N, THF, rt, 16 h; (f) (i) POCl3, CH3CN, reflux, 1 h; (ii) NaBH4, MeOH, 0 °C to rt, 16 h; (g) BrCH2CONHCH2Ph, iPr2EtN, Bu4NI, DMF, rt, 16 h; (h) CF3CH2I, Cs2CO3, DMF, 100 °C, 2 h.
