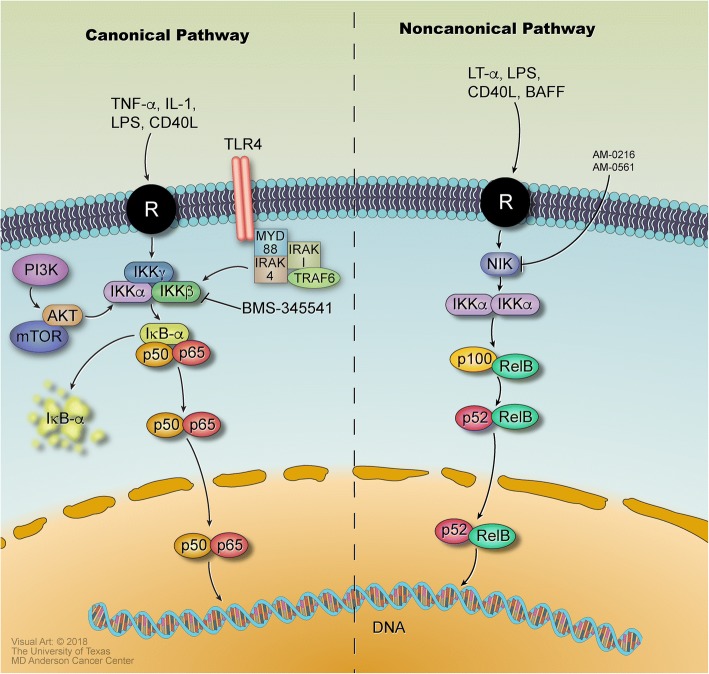
Fig. 2.
NF-κB signaling pathways with receptors, inhibitors, targets, and other molecules The canonical and non-canonical pathways for NF-κB signaling are mediated by various receptors and signaling molecules, including toll-like receptors (TLR), tumor necrosis factor receptors (TNFR), interleukin-1 receptor (IL-1R), CD40, initiation of B cell activation factor (BAFFR), lymphotoxin β- receptor (LTβR), and receptor activator for nuclear factor kappa B (RANK). The canonical NF-κB pathway involves the inhibition of NF-κB by IκB, which binds to the p50–p65 heterodimer in the cytoplasm and prevents it from entering the nucleus. Activation of BCR, TNFR, and IL-1R receptors initiates adapter protein and signaling kinase responses, leading to activation of the IκB kinase (IKK) complex. Kinases in the IKK complex phosphorylate IκB and lead to its poly-ubiquitination and proteasomal degradation. This allows the p50 and p65-RelA heterodimer (a complex from the NF-κB family) to be released into the nucleus to induce gene expression. In the non-canonical pathway, IKKα is activated by the upstream kinase NF-κB-inducing kinase (NIK), which promotes the processing of p100 into the active RelB-p52 isoform of NF-κB. NIK is downregulated by the expression of TRAF2 and TRAF3, which are negative regulators of non-canonical NF-κB signaling that interact with BIRC2 and BIRC3 [1]. Unlike the canonical pathway, the non-canonical pathway does not rely on IKKβ or IKKγ (NEMO); it only needs IKKα to phosphorylate the p52 precursor, p100