Abstract
Cucumber mosaic virus (CMV) causes great losses in Bhut Jolokia pepper (Capsicum chinense Jacq.) plantations in Assam, India. To investigate possible means to induce plant resistance against this virus, the crude extract of bacterially-expressed double-stranded (ds) RNA, derived from CMV-2b gene (dsRNA_CMV-2b), was exogenously applied along with CMV-G strain onto Bhut Jolokia plants. In this ‘RNA-vaccination’ bioassay, disease incidence, assessed by testing the plants at 21 days post inoculation by DAS-ELISA, ranged from 0 to 29% in case of dsRNA-treated plants, and from 55 to 92% when only CMV was applied. CMV-infected pepper plants became severely stunted, having dull light green foliage with leathery appearance, whereas plants receiving dsRNA_CMV-2b exhibited milder symptoms or remained healthy. The results obtained suggest that this non-transgenic approach has a considerable effect in protecting pepper against CMV.
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0452-6) contains supplementary material, which is available to authorized users.
Keywords: CMV, CMV-2b, Doubled stranded RNA, ELISA, RNA vaccine, RNAi
Bhut Jolokia pepper (Capsicum chinense Jacq.), well-recognized as one of the hottest chili pepper cultivars [3], is extensively grown in North Eastern region of India, predominantly in the states of Assam, Nagaland and Manipur [15] for its high capsaicin content (3%) and medicinal property apart from its culinary uses [15]. A Bhut Jolokia crop is threatened by a range of plant pathogens and among these, cucumber mosaic virus (CMV) causes severe crop damage, leading to low productivity [2]. In a study conducted in 2012–2014, CMV was detected by DAS-ELISA in 55% of the Bhut Jolokia fields in Assam state with plants exhibiting mosaic, crinkling, dwarfing and reduced leaf size [2]. The virus is transmitted to pepper by the aphid vector Aphis gossypii. However, traditional vector management strategies are not adequate in controlling CMV disease in the field [2]. Studies on developing transgenic crop resistance against plant viruses [16], including CMV, have been most intense in the last two decades. Several effective transgenic strategies based on pathogen-derived resistance have been identified; namely, resistance mediated by protein (e.g. the viral coat protein, viral movement protein) [7] or by RNA via post-transcriptional gene silencing (PTGS), a defense mechanism of eukaryotes against invasive nucleic acids [5].
Significant progress in the area of nucleic acid-based vaccines, i.e. molecules that could induce the plant defense machinery, has been achieved during the last few years. RNA silencing refers to a group of recently discovered mechanisms where dsRNA regulates eukaryotic gene expression in a sequence-specific manner [9]. Double-stranded RNA (dsRNA) is an inducer molecule of the RNA silencing pathway that is present in all higher eukaryotes and controls gene expression at the post-transcriptional level [9]. This mechanism allows the cell to recognize aberrant genetic material in a highly sequence specific manner [9]. Virus-derived dsRNAs could trigger the PTGS in plants. As a response to this defense mechanism, viruses have evolved or adapted genes to produce molecules, which could inhibit expression or inactivate components of the RNA silencing (RNA interference, RNAi) pathways [13, 16]. Introduction of dsRNA in eukaryotic cells could trigger RNAi against viruses in a sequence-specific manner [16]. More importantly, there are several examples where topical application of dsRNA induced plant resistance against the cognate virus (e.g. [17]).
For CMV, a member of the Bromoviridae family with a very wide host range and a tripartite genome, it is known that the 2b protein, encoded by RNA 2, has a long distance movement function [10, 13, 18] and was among the first silencing suppressors of PTGS identified [13]. CMV-2b protein is a symptom determinant and counter-defense factor that interferes with the plant’s PTGS pathway [18]. The RNA silencing suppressor activity of CMV-2b protein has been attributed to its nuclear or nucleolar localization [4], its slicer activity inhibition of Argonaute 1 (AGO1) [10] and AGO4 [4], and its ability to bind small RNAs (such as siRNAs) in vitro [6] and in vivo [4].
It has been reported previously that dsRNA derived from the CMV-2b gene (dsRNA_CMV-2b) and produced by enzymatic reactions in a test tube (‘in vitro’) or in bacterial cells (‘in vivo’), induced resistance against CMV upon its exogenous application in tobacco [8]. Therefore, it was hypothesized that dsRNA_CMV-2b can be utilized as an ‘RNA vaccine’ to induce resistance against CMV also in another host plant, i.e. Bhut Jolokia pepper. To test this hypothesis, Bhut Jolokia pepper seedlings were treated with CMV-G strain [8] in combination with the dsRNA_CMV-2b molecules and compared over a period of 60 days to plants treated only with CMV-G. Four replications of this bioassay were performed.
The dsRNA molecules used in the bioassay were produced in bacterial cells (‘in vivo’), since it is less expensive than the in vitro production, following the method described previously [8] with few modifications, as follows. The bacterial strain used was Escherichia coli (strain HT115 [DE3]), an RNAase III-deficient strain modified to express T7 RNA polymerase from an IPTG-inducible promoter. A PCR amplicon of 369 bp fragment of CMV-2b was cloned in the plasmid LITMUS28i (New England BioLabs, UK) and the construct was introduced into HT115 (DE3) cells. A selected transformant was grown in LB with the appropriate antibiotics and subcultured in 500 ml up to OD600 = 0.4. Then, addition of 1 mM IPTG induced T7 polymerase expression and production of dsRNA_CMV-2b in the bacterial cells. After a 4-h shaking incubation, bacterial cells were collected and total nucleic acids were extracted from them, following a crude extraction method with a standard phenol–chloroform step prior to ethanol precipitation, as previously described [8] but omitting DNAse I and RNase digestions. The nucleic acid pellet was resuspended in DEPC-treated water and constituted the ‘RNA vaccine’ used in the bioassay.
For the bioassay, seeds of Bhut Jolokia pepper were sown in 1.5-in. diameter pots containing peat moss media and kept in a growth chamber with controlled temperature and light. Once pepper seedlings emerged, they were transplanted individually to 1.5-in. diameter pots with the same growth medium. Plantlets of 31–38 days old were inoculated with CMV-G (treatment 1) or CMV-G in combination with dsRNA_CMV-2b (treatment 2). CMV inoculum consisted of leaf sap extracted from tobacco plants 14 days post inoculation (dpi) with CMV-G strain [8]. In treatment 1, CMV-containing sap was mixed with water (4:1 ratio), whereas in treatment 2, CMV-containing sap was mixed with dsRNA_CMV-2b (4:1 ratio). Application of these two mixtures onto plants was performed by rubbing carborundum-dusted leaf surfaces with 20 μl of the respective mixture per leaf. Each treatment was applied to 20 plants with two leaves per plant treated. Inoculated plants were maintained in a growth chamber under insect proof conditions at 22 °C day/night temperatures and 16 h daylight.
The Bhut Jolokia plants were visually inspected for CMV symptoms at 7, 14, 21 and 30 dpi, upon treatment 1 or 2, in order to estimate the infection percentage and infection progress rate. More specifically, symptoms were classified in three categories, namely: (a) blisters and deformed leaves, (b) chlorosis extended from basal portion of leaf and (c) mosaic symptoms. The symptom severity of CMV-infected Bhut Jolokia plants was estimated by a scoring system (ordinal disease rating scale) using an index ranging from 0 to 3, and defined as follows: 0 = No infection (none of the above-mentioned symptom categories is observed), 1 = Mild infection (any one of the three symptom categories is observed), 2 = Moderate infection (any two of the three symptom categories are observed) and 3 = Severe infection (all three symptom categories were observed). Furthermore, the impact of each treatment on plant growth was estimated at 30 dpi by measuring the plant height and leaf length of the second leaf from the top. Data were statistically analyzed by ANOVA using JMP8 software (JMP, USA) with significance level of 0.05. Finally, visual inspection of all vaccinated and non-vaccinated plants was extended until 60 dpi to record any irregular growth incidence.
Apart from the visual inspection of the treated plants, the percentage of CMV infected plants in the bioassay was also determined at 21 dpi by ELISA testing. From each plant, leaf tissue of fully expanded systemic young leaves (preferably second leaf from the top) was sampled using the lid of a sterile microcentrifuge tube to punch a leaf disk straight into the tube and brought to the laboratory under ice for ELISA testing. DAS-ELISA was performed using the ELISA kit Cucumber Mosaic Virus DTL complete kit (LOEWE®, DE) according to manufacturer’s instructions. ELISA results were recorded 1 h after substrate addition, using the microplate spectrophotometer Multiskan GO (Thermo Scientific, UK) at 405 nm. Positive and negative controls were added to each microtitre plate.
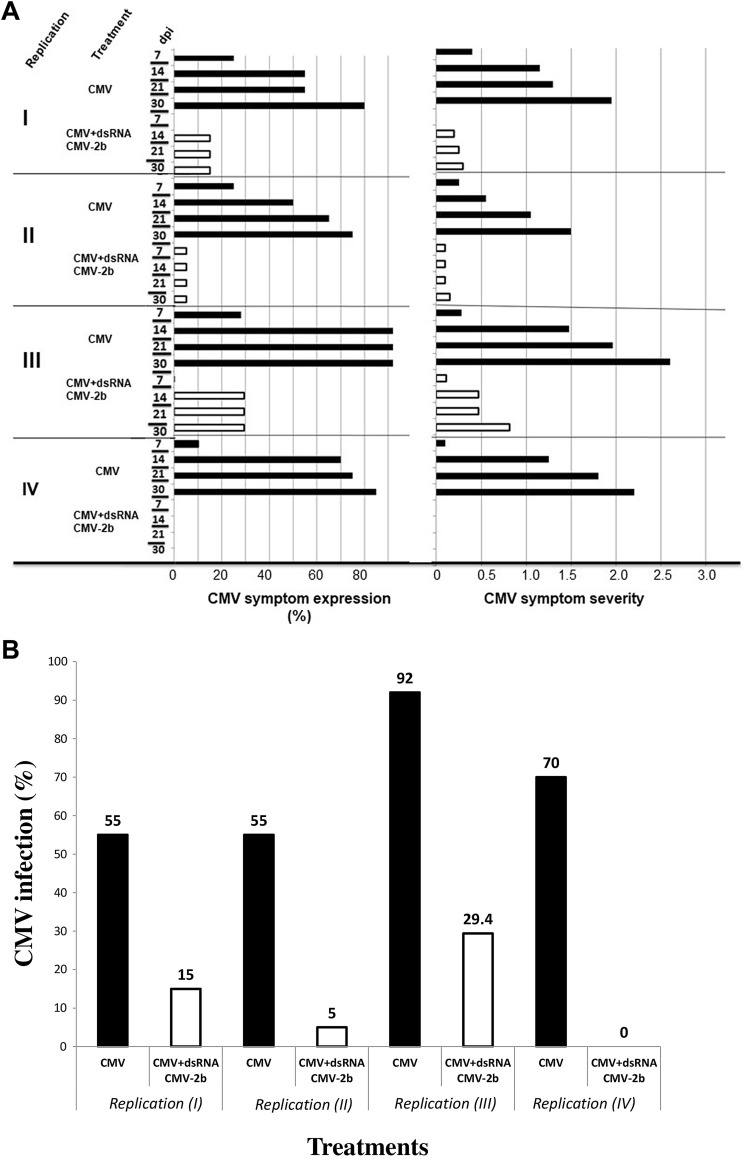
Plants inoculated with CMV-G exhibited a reduced overall growth after 35 days, in comparison to plants treated with CMV + dsRNA2b. In specific, for plants inoculated with CMV-G (‘treatment 1’), symptom expression was prominent after 14 dpi, though there had been a continuous increase in the percentage of plants exhibiting symptoms from 7 dpi onwards (Fig. 1a). Typical CMV symptoms on Bhut Jolokia were observed, namely chlorosis from the basal portion of the leaf, followed by blisters along with deformed leaves. Leaves showed greenish mosaic, which turned to chlorotic mosaic at later stages of infection. Finally, CMV infected plants were stunted with wrinkled leaves. Similar type of CMV symptomatology was observed previously [19] on chilli pepper plants.
Fig. 1.
Protection of Bhut Jolokia pepper plants against CMV upon application of dsRNA_CMV-2b. a Left: Progress of CMV symptom expression in CMV-treated (black bars) and CMV + dsRNA_CMV-2b-treated pepper plants (white bars) over time (7–30 dpi) based on visual plant inspection; right: progress of symptom severity in CMV-treated (black bars) and CMV + dsRNA_CMV-2b-treated pepper plants (white bars) over time (7–30 dpi) based on the scoring system described in the text and calculations using least mean square; dpi: days post inoculation. b Percentage of infected pepper plants treated with CMV or CMV + dsRNA_CMV-2b at 21 dpi, based on DAS-ELISA results. The numbers at the top of the bars indicate the respective CMV infection percentages
Plants inoculated with CMV in combination with dsRNA_CMV-2b (‘treatment 2’) showed a lower percentage of infection (Fig. 1a) that did not increase after 14 dpi and less severe symptoms (Fig. 1a). Symptom severity had a lower score index, which ranged between time points 7 and 30 dpi as follows: 0–0.3 (replication I), 0.1–0.15 (replication II), 0.11–0.82 (replication III) and 0 (replication IV) (Fig. 1b; Table 1). The symptom severity upon CMV infection showed a variation between time points 7 and 30 dpi as follows: 0.4–1.95 (replication I), 0.25–1.5 (replication II), 0.28–2.6 (replication III) and 0.1–2.2 (replication IV) (Fig. 1a). In addition, an obvious delay in symptom expression in ‘treatment 2’ was observed (Fig. 1a).
Table 1.
Leaf length and plant height increase measurements of the vaccinated and non-vaccinated Bhut Jolokia pepper plants of the bioassay at 30 dpi
| Replication No. | Leaf lengtha,b (cm) | Increase in plant heightb (cm) | ||
|---|---|---|---|---|
| CMV | CMV + dsRNA_CMV-2b | CMV | CMV + dsRNA_CMV-2b | |
| I | NR | NR | 0.12 ± 0.17 | 1.7 ± 0.17 |
| II | 3.57 ± 0.17 | 5.43 ± 0.17 | 0.27 ± 0.12 | 1.03 ± 0.12 |
| III | 3.54 ± 0.15 | 5.61 ± 0.19 | 0.12 ± 0.1 | 1.21 ± 0.12 |
| IV | 3.3 ± 0.13 | 5.8 ± 0.13 | 0.675 ± 0.12 | 3.53 ± 0.12 |
NR not recorded
aMeasurements refer to the second leaf from the top measured at 30 dpi
bMean ± standard error of values recorded at 30 dpi
Furthermore, based on DAS-ELISA results at 21 dpi, the percentage of infected plants ranged in the four bioassay replications from 55 to 92% in the case of plants inoculated with CMV (‘treatment 1’), and from 0 to 29% in the case of plants treated with CMV + dsRNA_CMV-2b (‘treatment 2’) (Fig. 1b). Statistical analysis by t test of the ELISA results obtained in the four replications showed significant difference between the two treatments [t(6) = 5.11, p = 0.002 (2 tail)]. These results suggested that the exogenously applied dsRNA_CMV-2b has a considerable effect in controlling CMV infection.
Other parameters compared between the two treatments were the plant height and the leaf size. In all bioassay replications plants receiving dsRNA_CMV-2b exhibited better growth as indicated by a higher increase in the leaf length and plant height, as compared to those that did not receive it (Table 1). In addition, flowering of vaccinated plants occurred at 55 dpi, whereas the non-vaccinated plants appeared stunted and had non-reproductive growth habit up to 60 dpi.
In conclusion, our results suggest that the exogenous application onto Bhut Jolokia pepper plants of a crude extract of bacterially-expressed dsRNA molecules derived from CMV-2b gene, caused significant reduction in percentage of CMV infection, and allowed a better vegetative plant growth. The research conducted using the CMV-G strain and Bhut Jolokia pepper plants serves as a proof of concept of the RNA vaccination strategy. The CMV-G strain is a severe strain, well characterized at molecular and virulence level [12], causing characteristic symptoms onto Bhut Jolokia plants. It belongs to subgroup I, a category associated before with disease outbreaks in Bhut Jolokia fields in India and elsewhere or with germplasm screening projects [1, 11, 14]. This vaccination strategy could further be tested against other CMV strains obtained from infected Bhut Jolokia crops in Assam and North East India, where the virus occurs and causes considerable crop losses [2]. Although the molecular mechanism underlying the induction of virus resistance was not studied in the present study, it is likely that the application of dsRNA of the 2b gene, a known silencing suppressor, triggers the RNAi plant defense mechanism leading to reduced disease incidence and symptom severity. This hypothesis is to be addressed in future experiments. The RNA-based vaccination approach reported here could enhance effectiveness of plant protection schemes for Bhut Jolokia pepper plants in nurseries and fields against CMV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Munmi Borah was supported by a fellowship from European Commission (Erasmus Mundus BRAVE, Grant 2013-2536). Mr Anastasios Katsileros is acknowledged for his assistance in the statistical analysis of the data with JMP. The dsRNA molecules were produced in the frame of a bilateral project between Greece and China (‘sRNAvac’, Grant 12CHN116) funded by the General Secretariat for Research and Technology (GSRT) in Greece.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0452-6) contains supplementary material, which is available to authorized users.
References
- 1.Badu B, Dankers H, Paret ML. First report of Cucumber mosaic virus associated with Capsicum chinense var. Scotch Bonnet in Florida. Plant Dis. 2014;98:1016. doi: 10.1094/PDIS-12-13-1276-PDN. [DOI] [PubMed] [Google Scholar]
- 2.Baruah BR, Kashyap A, Nath PD. Incidence, detection and integrated management of viral disease complex in Bhut Jolokia, a chilli cultivar in Assam. Ann Plant Prot Sci. 2016;24:136–141. [Google Scholar]
- 3.Bosland PW, Baral JP. Bhut Jolokia—the world’s hottest known chilli is a putative naturally occurring inter specific hybrid. HortScience. 2007;42:222–224. [Google Scholar]
- 4.Canto T, Prior DAM, Hellwald K-H, Oparka KJ, Palukaitis P. Characterization of Cucumber Mosaic Virus. IV. Movement protein and coat protein are both essential for cell-to-cell movement of Cucumber mosaic virus. Virology. 1997;237:237–248. doi: 10.1006/viro.1997.8804. [DOI] [PubMed] [Google Scholar]
- 5.Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–553. doi: 10.1016/S0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- 6.Ding SW, Li WX, Symons RH. A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 1995;14:5762–5772. doi: 10.1002/j.1460-2075.1995.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González I, Martínez L, Rakitina DV, Lewsey MG, Atencio FA, Llave C, Kalinina NO, Carr JP, Palukaitis P, Canto T. Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to RNA silencing suppressor activity. Mol Plant Microbe Interact. 2010;23:294–303. doi: 10.1094/MPMI-23-3-0294. [DOI] [PubMed] [Google Scholar]
- 8.Holeva MC, Sclavounos AP, Milla SP, Kyriakopoulou PE, Voloudakis AE. External application of dsRNA of the capsid protein (CP) or 2b gene of CMV reduces the severity of CMV-infection in tobacco. In: ΧΙΙΙ International Congress on molecular plant–microbe interactions, 21–27 July 2007, Sorrento, Italy.
- 9.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery MK, Xu S, Fire A. RNA as a target of double-stranded RNA-mediated genetic interference in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95:15502–15507. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naresh P, Reddy MK, Reddy PHC, Reddy KM. Screening chilli (Capsicum spp.) germplasm against Cucumber mosaic virus and Chilli veinal mottle virus and inheritance of resistance. Eur J Plant Pathol. 2016;146:451–464. doi: 10.1007/s10658-016-0930-x. [DOI] [Google Scholar]
- 12.Sclavounos AP, Voloudakis AE, Arabatzis C, Kyriakopoulou PE. A severe hellenic CMV tomato isolate: symptom variability in tobacco, characterization and discrimination of variants. Eur J Plant Pathol. 2006;115:63–172. doi: 10.1007/s10658-006-0010-8. [DOI] [Google Scholar]
- 13.Sijen T, Kooter JM. Post-transcriptional gene-silencing: RNAs on the attack or on the defense? BioEssays. 2000;22:520–531. doi: 10.1002/(SICI)1521-1878(200006)22:6<520::AID-BIES5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Kuroda T, Miura Y, Murai J. Screening and field trials of virus resistant sources in Capsicum spp. Plant Dis. 2003;87:779–783. doi: 10.1094/PDIS.2003.87.7.779. [DOI] [PubMed] [Google Scholar]
- 15.Talukdar J, Saikia AK, Borah P. Survey and detection of the diseases of Bhut Jolokia (Capsicum chinense Jacq.) in Assam. J Crop Weed. 2015;11:186–192. [Google Scholar]
- 16.Vance V, Vaucheret H. RNA silencing in plants-defense and counterdefense. Science. 2001;292:2277–2280. doi: 10.1126/science.1061334. [DOI] [PubMed] [Google Scholar]
- 17.Voloudakis AE, Holeva MC, Sarin LP, Bamford DH, Vargas M, Poranen MM, Tenllado F. Efficient double-stranded RNA production methods for utilization in plant virus control. Mol Plant Microbe Interact. 2015;1236:255–274. doi: 10.1007/978-1-4939-1743-3_19. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006;20:3255–3268. doi: 10.1101/gad.1495506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitter TA, Murphy JF. Cucumber mosaic. Plant Health Instr. 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.