Scheme 2.
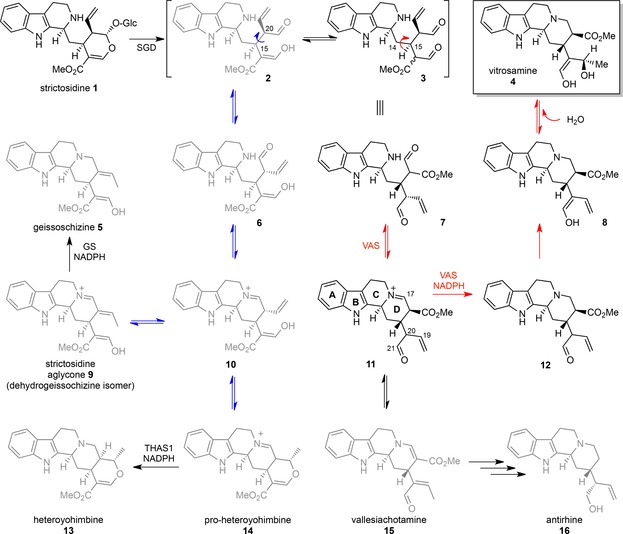
Rearrangement of strictosidine aglycone to give rise to different MIA structural classes. Rotation around the C‐15−C‐20 bond (blue arrow) and subsequent cyclization of ring D give rise to the heteroyohimbine and geissoschizine backbones. Rotation around the C‐14−C‐15 bond (red arrow) and subsequent cyclization of ring D yields the substrate of Cro013448, leading to vallesiachotamine (15), antirhine (16, previously observed natural products) and vitrosamine (4).
