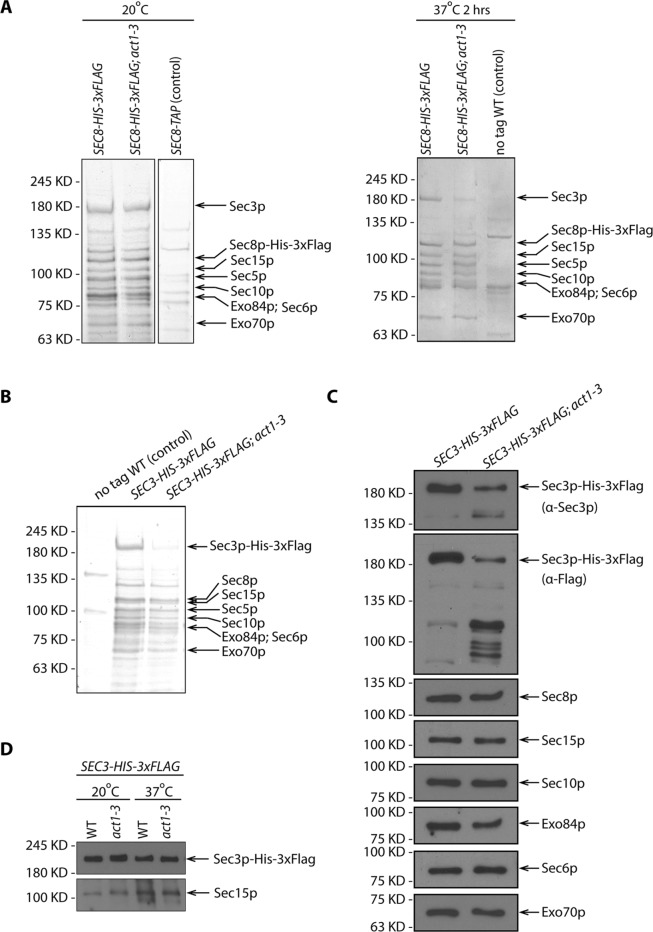
FIGURE 8:
Sec3p is unstable in act1-3 lysate. (A) SDS–PAGE and Coomassie staining of exocyst complexes purified using Sec8p-His-3xFlag from WT and act1-3 mutant yeast lysates (NY3271 and NY3272). Left panel shows the purification from yeast strains growing at permissive temperature 20°C. Right panel shows the purification from yeast strains shifted to nonpermissive temperature 37°C for 2 h before harvesting. WT strains with Sec8p-TAP tagged or Sec8p untagged were used as negative controls respectively. For comparison, the loading ratio for the purified samples from WT and act1-3 is 1:1. (B) SDS–PAGE and Coomassie staining of exocyst complexes purified with Sec3p-His-3xFlag from WT and act1-3 mutant yeast strains (NY3273 and NY3274). For comparison, the loading ratio for the purified samples from WT and act1-3 is 1:2. (C) Western blot analysis of immunoisolated exocyst with Sec3p-His-3xFlag from WT and act1-3 mutant yeast strains (NY3273 and NY3274) on a 6% SDS–PAGE gel. Proteins were detected with anti-Flag, anti-Sec3p, anti-Sec8p, anti-Sec15p, anti-Sec10p, anti-Exo84p, anti-Sec6p, and anti-Exo70p antibodies. For comparison, the loading ratio for the purified samples from WT and act1-3 is 1:2. (D) Total cell lysates by quick NaOH lysis from WT and act1-3 mutant yeast strains were analyzed by Western blot with anti-Flag antibody and anti-Sec15p serum.