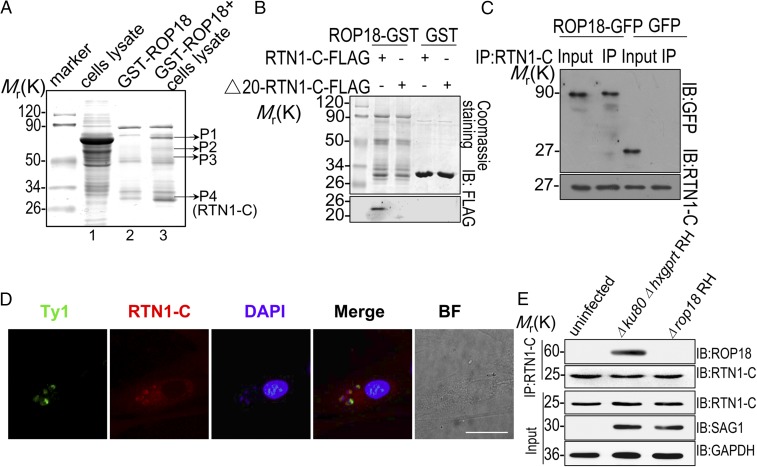
Fig. 2.
ROP18 interacts with RTN1-C. (A) Mass spectrometry screening to identify ROP18-binding proteins. ROP18-binding proteins were isolated from human SH-SY5Y neuroblastoma cell line extracts. Silver-stained SDS-polyacrylamide gels of control (lane 2) and ROP18-GST pulldown complexes (lane 3) are presented. Molecular weight markers are indicated. Bands present in the ROP18-GST pulldown were excised (bands shown), and protein determination was performed by MALDI-TOF mass spectrometry. (B) Confirmation of the binding site of RTN1-C and ROP18 by an in vitro GST pulldown assay. ROP18-GST bound to glutathione beads was used as an affinity matrix to absorb FLAG-tagged full-length RTN1-C and its truncate Δ20-RTN1-C–expressed Neuro2a cells. IB, immunoblot. (C) Verifying the interaction of RTN1-C with ROP18 through immunoprecipitation. Neuro2a cells were transfected with ROP18-GFP or control GFP vector for 24 h. Starting fractions (Input) and immunoprecipitates (IP) were analyzed by SDS/PAGE and Western blotting using GFP and RTN1-C antibodies. The result indicates that ROP18 (Upper) and RTN1-C (Lower) were coimmunoprecipitated. (D) Primary cortical neuronal cells grown on glass coverslips were infected with the ROP18-WT-Ty1 RH strain at a multiplicity of infection (m.o.i.) of 1 for 24 h. Double immunofluorescence was performed using mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti–RTN1-C (red) antibodies. A distinctive pattern of RTN1-C and ROP18 localization was observed around the parasite. BF, bright field. (Scale bar, 5 μm.) (E) ROP18-expressing transgenic strains were used to determine ROP18 and RTN-1C interaction. The SH-SY5Y cells were infected with the indicated parasites at an m.o.i. of ∼1. At 12 h after infection, immunoprecipitation (IP) of RTN-1C from infected cell lysates was detected with rabbit polyclonal ROP18 antibody.