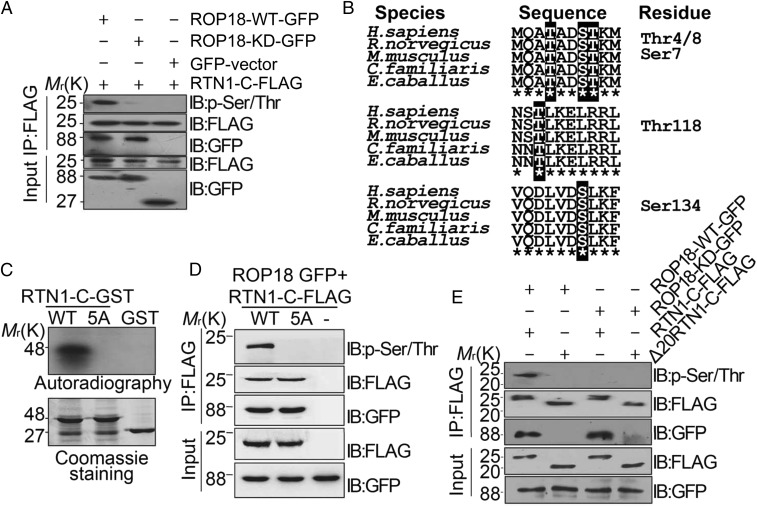
Fig. 3.
Ser7/134 and Thr4/8/118 on RTN1-C are phosphorylation sites for ROP18 in vitro and in vivo. (A) Neuro2a cells were cotransfected with ROP18-WT-GFP or ROP18-KD-GFP and FLAG-tagged RTN1-C for 24 h, and the cell lysates were immunoprecipitated (IP) with FLAG antibodies. RTN1-C phosphorylation was detected by the phospho-Ser/Thr antibody. IB, immunoblot. (B) Three peptides containing putative phosphorylation sites are indicated with red letters. ClustalW alignment of the amino acid sequences from 1–10, 116–125, and 128–137 of the human RTN1-C with rat, mouse, dog, and horse sequences. (C) ROP18 phosphorylates RTN1-C Ser7/134 and Thr4/8/118 in vitro. Bacterially expressed GST-tagged wild-type RTN1-C and RTN1-C-5A mutant (SS7/134AA + TTT4/8/118AAA) were purified on glutathione-agarose beads and phosphorylated in vitro using [32P] ATP and eukaryotically expressed ROP18 as described (17). Samples were separated by SDS/PAGE gel (Lower) and subsequently incubated with X-ray film (Upper). Of note, in the presence of ROP18, dramatic incorporation of 32P into wild-type RTN1-C, but not RTN1-C-5A, mutant protein was observed. (D) Wild-type or mutant FLAG-tagged RTN1-C-5A was transiently coexpressed with ROP18-GFP in Neuro2a cells for 24 h and immunoblotted with phospho-Ser/Thr antibody and the indicated antibodies, respectively. (E) FLAG-tagged full-length RTN1-C and its truncated form Δ20-RTN1-C were transiently coexpressed with ROP18-WT-GFP or ROP18-KD-GFP in Neuro2a cells for 24 h. Whole-cell extracts were immunoblotted with phospho-Ser/Thr antibody and the indicated antibodies, respectively.