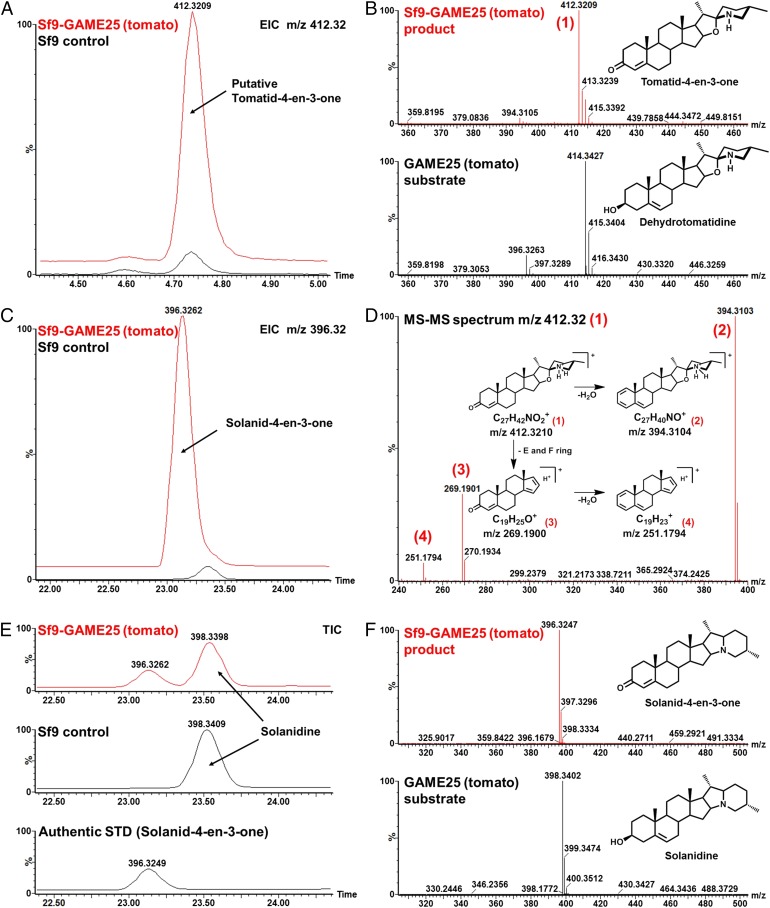
Fig. 5.
Activity of recombinant tomato GAME25 produced in insect cells. (A) Overlay of extracted ion chromatograms of m/z 412.32 Da, [M + H+]+ (mass of the GAME25 reaction product), and the control reaction obtained with dehydrotomatidine as a substrate. (B) Mass spectra and structures of the detected product (Upper) and substrate (Lower) of the GAME25 enzymatic reaction with dehydrotomatidine as substrate. (C) Overlay of extracted ion chromatograms of m/z 396.32 Da, [M + H+]+ (mass of the GAME25 reaction product), and the control reaction with solanidine as substrate. (D) Mass fragmentation spectrum of the GAME25 enzymatic reaction product (with dehydrotomatidine as substrate), including the interpretation of the detected mass fragments. The fragmentation pattern corresponds to the tomatid-4-en-3-one (proposed structure of the GAME25 product). (E) Chromatograms of the GAME25 enzymatic reaction (Upper), control reaction (Middle), both with solanidine as substrate, and the solanid-4-en-3-one authentic standard (Lower). The newly formed product (at retention time 23.2 min) coeluted with the solanid-4-en-3-one commercial authentic standard. Comparison of MS-MS spectra between the newly formed product and authentic standard solanid-4-en-3-one was similar and is provided in SI Appendix, Fig. S17. Thus, this newly formed GAME25 product was assigned as solanid-4-en-3-one. (F) Mass spectra and structures of the detected product (Upper) and substrate (Lower) of the GAME25 enzymatic reaction with solanidine as substrate. Analysis of enzyme assay reactions was carried out by LC–MS. The control reaction was performed using protein extracts from nontransfected Sf9 insect cell microsomes. EIC, extracted ion chromatogram; m/z, mass to charge; STD, metabolite standard; TIC, total ion chromatogram.