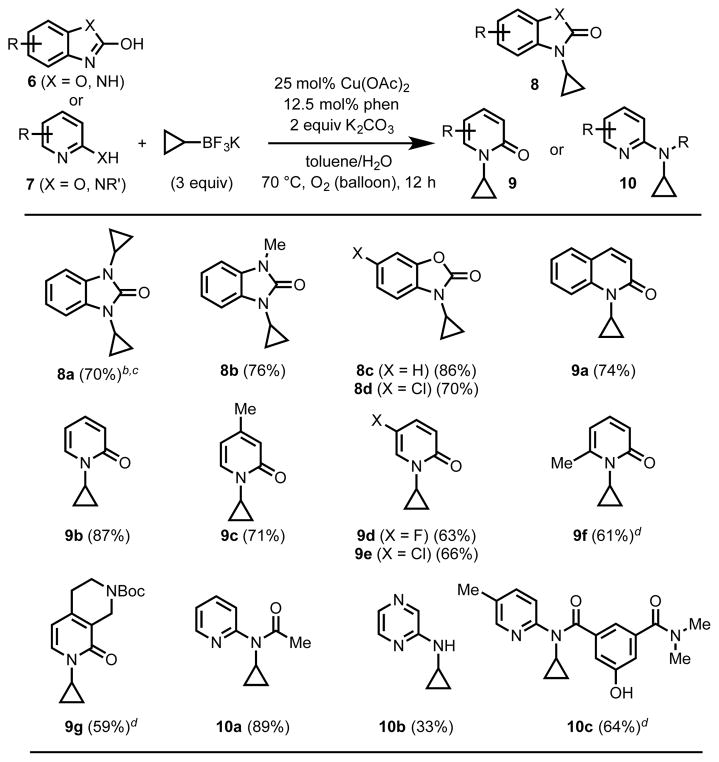
Table 3.
Substrate scope of azaheterocycle N-cyclopropylation.a
All reactions were run on a 0.3–0.6 mmol scale. Percentages correspond to isolated yields.
13% of the mono-cyclopropylated product (8a′) was also isolated.
With 10% Cu(OAc)2 and 10% phen under otherwise identical conditions: 49% 8a (bis), 15% 8a′ (mono). The connectivity of 8a′ was confirmed by single-crystal X-ray diffraction (see SI).
25 mol% Cu(OAc)2, 25 mol% phen.