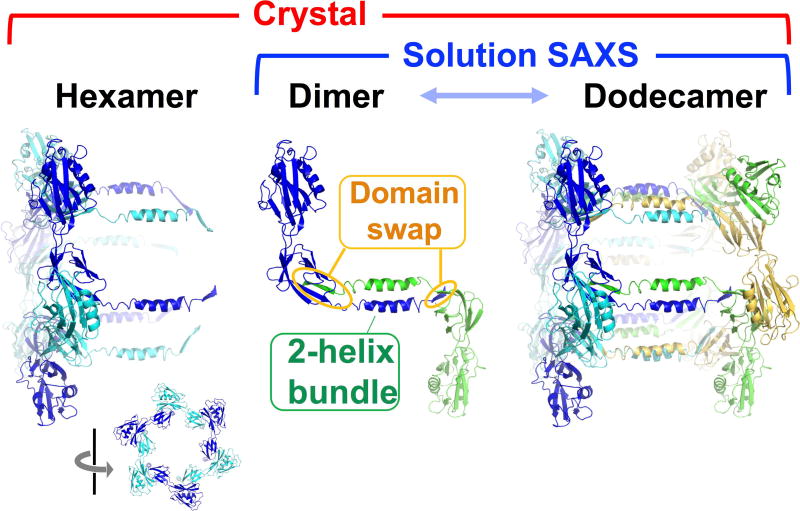
Figure 4. Determination of Multimerization in Solution by SAXS.
Based on the crystallographic lattice and electron microscopy images, the crystallographers assigned FliD (Ts0886) as a hexamer. However, the molecular weight calculated from the SAXS data of the same sequence suggested that FliD was a dimer. Comparison of all possible dimers in the crystallographic lattice identified one as the most likely dimer. The involvement in domain swapping and in a two-helix bundle supported this dimer as biologically relevant. Another construct of FliD showed strong concentration dependence of assembly (dimer to dodecamer) and thus was not included as a target in CASP. We postulate that the dodecamer, observed also in the crystallographic lattice, is created from assembly of the dimeric form.