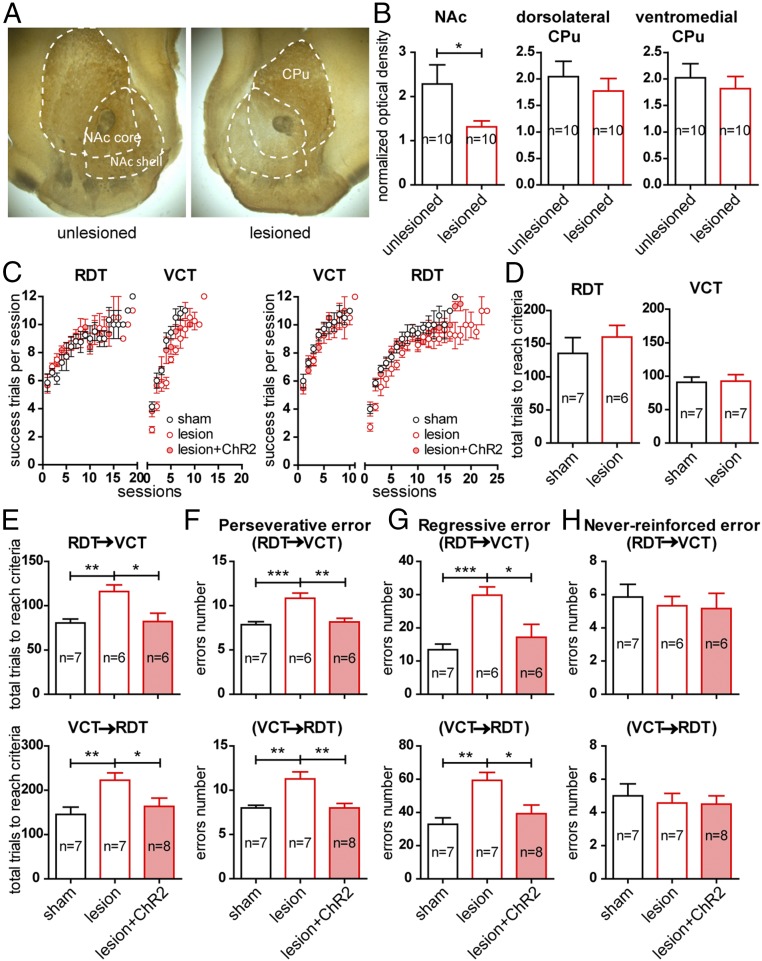
Fig. 3.
Focal dopamine depletion in the NAc impairs strategy-switching flexibility, and the impairment can be rescued by optogenetic activation of the PrL–NAc pathway. (A) A typical section showing that TH immunoreactivity was reduced in the NAc core and shell regions 2 wk after focal injection of 6-OHDA into the NAc. CPu, caudate putamen. (B) Normalized data of optical density in the NAc (Left), dorsolateral CPu (Middle), and ventromedial CPu (Right) from 10 animals. *P < 0.05, paired t test. (C) Tracking of success trials in RDT and VCT learning as well as their switching in the sham, 6-OHDA lesioned, and lesioned+ChR2 groups. (D–H) Pooled data suggest that NAc dopamine depletion did not affect the ability of the animals to acquire the RDT and the VCT (D). However, in the RDT–VCT and VCT–RDT switching tests, NAc dopamine depletion resulted in a significantly higher number of trials before reaching the criterion (E), accompanied by increases in perseverative errors (F) and regressive errors (G), but not in never-reinforced errors (H). Optogenetic activation of ChR2 of the PrL–NAc pathway in the lesioned mice restored switching performance to that in the sham group. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.