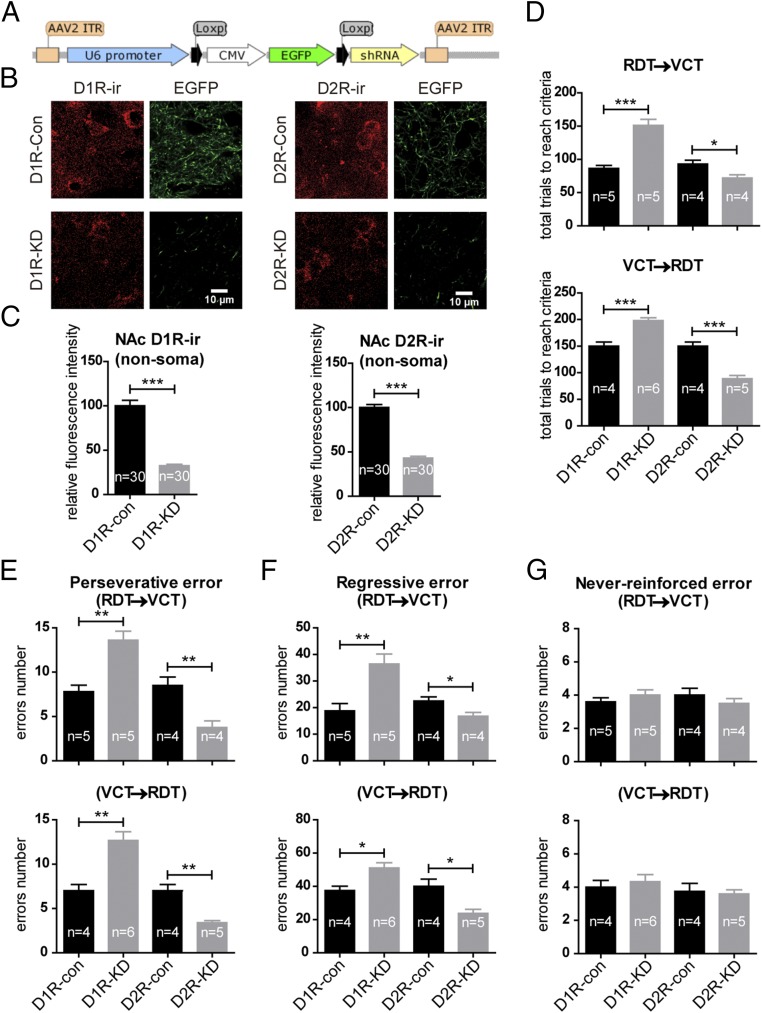
Fig. 7.
Knockdown of D1 receptors and D2 receptors in the PrL respectively impairs and improves task-switching flexibility. (A) Schematic of the construction of cre-dependent shRNA. (B) Typical images showing that 3 wk after AAV-shRNA injection (control) or AAV-shRNA injection in combination with prior AAV-hSyn-Cre injection (knockdown), D1R immunoreactivity (D1R-ir) or D2R immunoreactivity (D1R-ir) in the NAc was reduced in D1R-knockdown (D1R-KD) or D2R-knockdown (D2R-KD) groups, respectively, compared with the corresponding control (Con) groups. These reductions were in parallel with decreased EGFP fibers in the NAc due to the excision of the EGFP reporter by cre-recombinase present in the knockdown groups. (C) Quantification of the fluorescence intensity of D1R immunoreactivity or D2R immunoreactivity from the NAc nonsoma area revealed a significant reduction in the D1R-knockdown or D2R-knockdown groups compared with the respective controls. Each group was quantified from 30 randomly chosen regions of interest from two mice. (D) In both the RDT–VCT and the VCT–RDT switching paradigms, mice with D1R knockdown and D2R knockdown in PrL neurons respectively increased and decreased the total number of trials needed to reach the criterion compared with the respective controls. (E and F) In these animals, the number of perseverative errors (E) and regressive errors (F) committed changed in parallel with the total number of trials needed to reach the criterion. (G) Never-reinforced errors were not altered following the knockdown of either receptor. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.