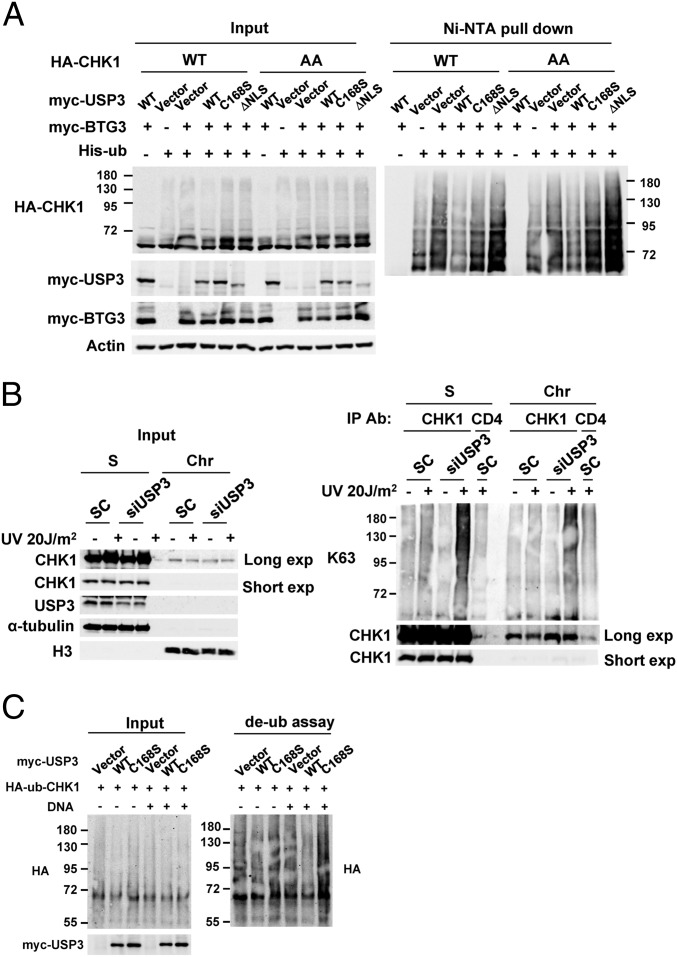
Fig. 3.
USP3 regulates CHK1 ubiquitination through its catalytic activity and nuclear localization. (A) BTG3-promoted CHK1 ubiquitination was decreased by its coexpression with WT USP3 but not with the C168S or ΔNLS mutants. In vivo ubiquitination was performed in 293T cells transfected with the indicated constructs, and the products were pulled down using Ni-NTA beads. AA, CHK1 mutant with Ser317 and Ser345 mutated to Ala. (B) K63-linked ubiquitination of endogenous CHK1 was increased by USP3 knockdown in HCT116 cells. Ubiquitination of CHK1 in soluble (S) and chromatin (Chr)-enriched fractions was analyzed by immunoprecipitation with anti-CHK1 antibody, followed by Western blotting using K63-linked specific ubiquitin antibody. (C) WT USP3, but not the C168S mutant, deubiquitinated CHK1 in vitro. The in vitro deubiquitination assay was performed by coincubating the purified ubiquitinated CHK1 and immunoprecipitated USP3 in the presence or absence of DNA.