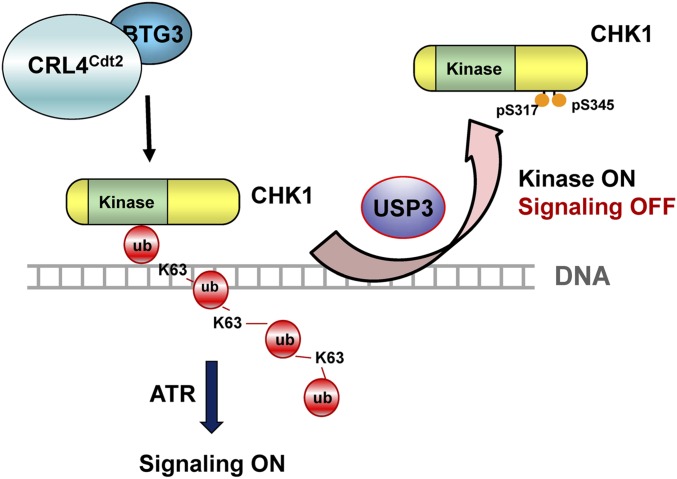
Fig. 7.
Model depicting the role of USP3 in the regulation of CHK1 in the DDR. Upon DNA damage, USP3 binds with CHK1 on chromatin, likely through the K63-linked ubiquitin chain on CHK1. By removing the ubiquitin chain from CHK1, USP3 releases CHK1 from chromatin, preventing its further phosphorylation and activation by upstream kinases. Concordantly, USP3 also renders CHK1 more accessible to its substrates by removing the ubiquitin chain that blocks the kinase active site.