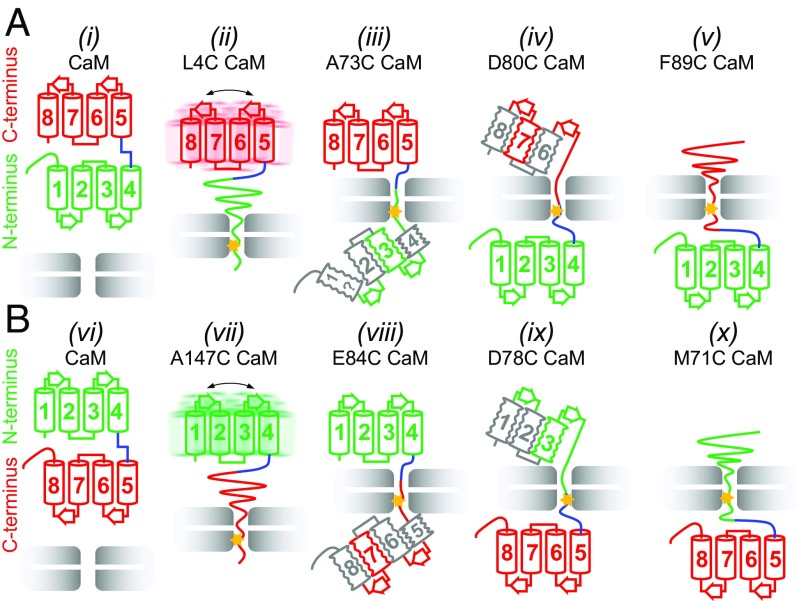
Fig. 6.
Cooperativity of domain unfolding depends on pulling direction. (A) Schematic of key unfolding steps for a pulling reaction starting from the N terminus of CaM. The N-lobe unfolds cooperatively (ii, attachment at position 4 indicated by star) and begins to refold before helix 4 is completely released from the unfoldase (iii, attachment at 73). Unfolding of helix 5 (iv, attachment at 80) precedes complete unfolding of the C-lobe (v, attachment at 89) that only refolds upon release of substrate. (B) Schematic of unfolding starting from the C terminus of CaM. The C-lobe unfolds cooperatively (vii, attachment at 147), with formation of a native-like intermediate before helix 5 is completely released (viii, attachment at 84). Attachment at position 78 (ix) leads to unfolding of helix 4 before cooperative unfolding of the remaining helices (x). Note that in A the N-lobe unfolds cooperatively, with the C-lobe unfolding in steps and the reverse occurs in B. Helices are colored in green or red when there are many probes indicating a native-like element, in gray when the evidence is indirect, and in gray with the symbol “?” when there is no evidence. Squiggles indicate the likely presence of conformational heterogeneity. In ii and vii the overall dynamics of C- and N-domains are only moderately affected by attachment to VAT.