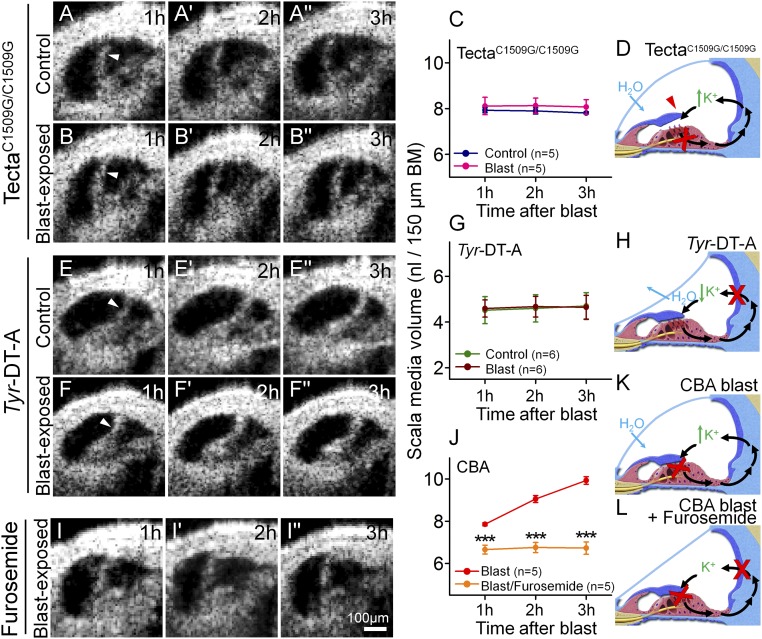
Fig. 4.
Stereociliary trauma and endolymphatic K+ are necessary for the development of endolymphatic hydrops after blast exposure. (A) A representative unexposed TectaC1509G/C1509G mouse demonstrates a stable, but increased, volume of endolymph. (B) A representative TectaC1509G/C1509G mouse after blast exposure. (C) Endolymph volume was higher than normal, yet unchanged by blast-exposure in TectaC1509G/C1509G mice. (D) Postulated mechanism of endolymph volume dysregulation in TectaC1509G/C1509G mice. The TM is elevated off the epithelium and so does not cause any static displacement of the OHC stereociliary bundles. Thus, K+ bias currents through transduction channels (red X) are reduced, increasing endolymph [K+]. H2O enters endolymph because of the increased osmotic load. (E) A representative unexposed Tyr-DT-A mouse demonstrates a stable, but decreased, volume of endolymph. (F) A representative Tyr-DT-A mouse after blast exposure. (G) Endolymph volume in Tyr-DT-A mice was lower than normal, yet unchanged, by blast exposure. (H) Postulated mechanism of endolymph volume dysregulation in Tyr-DT-A mice. K+ secretion from the stria vascularis is blocked (red X), reducing endolymph [K+]. H2O leaves endolymph because the osmotic load is now higher in perilymph. (I) A representative CBA mouse that was dosed with furosemide and then exposed to a blast. (J) Endolymph volume did not increase after blast exposure in mice pretreated with furosemide. (K) Postulated mechanism of endolymph volume dysregulation in CBA mice after blast exposure. Damaged stereocilia and OHC loss (red X) reduce K+ bias currents through transduction channels (red X), increasing endolymph [K+] and drawing in H2O. (L) With furosemide treatment, K+ secretion by the stria is also blocked, so endolymphatic [K+] is expected to remain stable. ***P < 0.001.