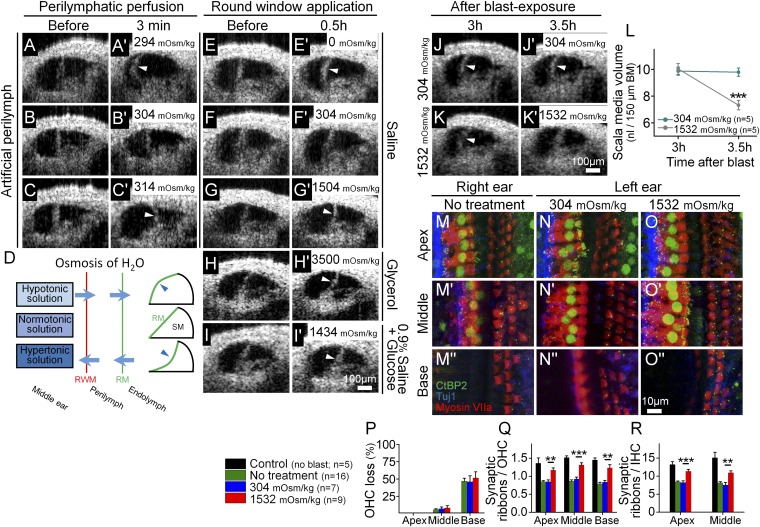
Fig. 5.
Osmotic treatment of endolymphatic hydrops partially rescues synaptic ribbon loss after blast exposure. (A–C) Perilymph osmolality modulates endolymph volume. Perfusion of hypotonic (A), normotonic (B), or hypertonic (C) artificial perilymph dynamically shifted the position of Reissner’s membrane (arrowheads). This occurred rapidly (<3 min). (D) Simple model of noninvasive osmotic challenge via the round window membrane (RWM) to alter the volume of endolymph and shift Reissner’s membrane (RM). Hypotonic solution applied to the middle ear causes endolymphatic hydrops, whereas hypertonic solution reduces endolymphatic volume. (E–I) The application of solutions of varying osmotic loads into the middle ear adjacent to the round window modulates endolymph volume. Representative OCT images were taken before and 30 min after applying the osmotic challenge. Hypotonic challenge (E) increased endolymph volume, normotonic saline (F) caused no change in endolymph volume, and hypertonic challenges (G–I) decreased endolymph volume. (J and K) Representative mice were treated with normotonic or hypertonic challenge 3 h after blast exposure. Repeat images were taken 30-min later. (L) Normotonic artificial perilymph had no impact on posttraumatic endolymphatic hydrops, whereas hypertonic artificial perilymph normalized endolymphatic volume. (M–O) The cochlear epithelium from representative mice 2 mo after blast exposure. Mice had either no treatment (M), normotonic artificial perilymph application to the middle ear after the blast (N), or hypertonic artificial perilymph application to the middle ear after the blast (O). Immunolabeling was done to visualize synaptic ribbons in hair cells (CtBP2), hair cells (myosin VIIa), and auditory neurons (Tuj1). (P–R) Quantification of OHC loss and synaptic ribbons per OHC and IHC. Hypertonic artificial perilymph reduced the loss of synaptic ribbons but did not affect the degree of OHC loss. **P < 0.01, ***P < 0.001.