Figure 3. Lipid droplet interactome with other organelles.
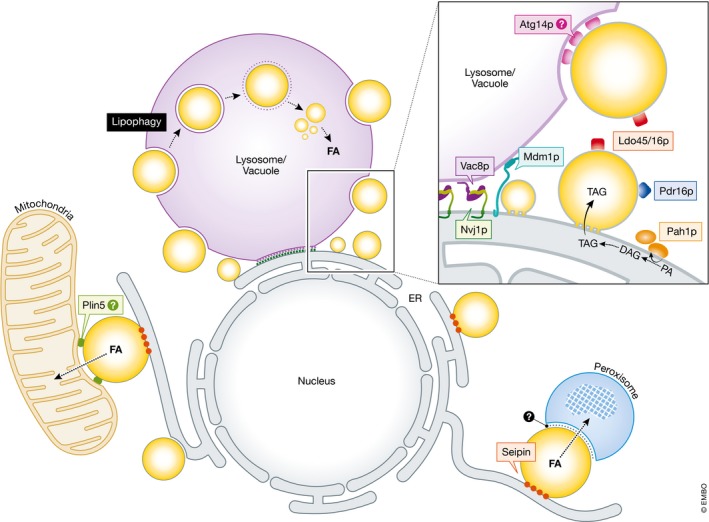
Droplets form contacts with numerous other organelles, including the endoplasmic reticulum (ER) via the protein seipin (red). Mitochondria–droplet contacts promote the transfer of fatty acids (FA) for β‐oxidation in mitochondria, and these contacts may be stabilized by perilipin 5 (Plin5, green). Similarly, in yeast peroxisome–droplet contacts are sites of FA transfer. Also in yeast, nuclear ER–vacuole junctions (NVJ) are sites of droplet budding. NVJ contact sites are maintained by the tethering proteins Nvj1p (green) and Vac8p (purple). NVJ‐associated droplets co‐localize with the NVJ tether Mdm1 (black), and the droplets are decorated with specific proteins including Prd16p (pink) and Ldo45p/16p (aqua). The PA phosphatase Pah1p (orange), which generates DAG from PA, is enriched at the NVJ periphery during the diauxic shift. NVJ‐derived droplets may be eventually digested via lipophagy at sterol‐rich microdomains at the vacuole surface in an ATG14‐dependent (gray) manner.
