Abstract
Background and Purpose
Cannabidiol (CBD) represents a promising therapeutic tool for treating cannabis use disorder (CUD). This study aimed to evaluate the effects of CBD on the behavioural and gene expression alterations induced by spontaneous cannabinoid withdrawal.
Experimental Approach
Spontaneous cannabinoid withdrawal was evaluated 12 h after cessation of CP‐55,940 treatment (0.5 mg·kg−1 every 12 h, i.p.; 7 days) in C57BL/6J mice. The effects of CBD (5, 10 and 20 mg·kg−1, i.p.) on withdrawal‐related behavioural signs were evaluated by measuring motor activity, somatic signs and anxiety‐like behaviour. Furthermore, gene expression changes in TH in the ventral tegmental area, and in the opioid μ receptor (Oprm1), cannabinoid CB1 receptor (Cnr1) and CB2 receptor (Cnr2) in the nucleus accumbens, were also evaluated using the real‐time PCR technique.
Key Results
The administration of CBD significantly blocked the increase in motor activity and the increased number of rearings, rubbings and jumpings associated with cannabinoid withdrawal, and it normalized the decrease in the number of groomings. However, CBD did not change somatic signs in vehicle‐treated animals. In addition, the anxiogenic‐like effect observed in abstinent mice disappeared with CBD administration, whereas CBD induced an anxiolytic‐like effect in non‐abstinent animals. Moreover, CBD normalized gene expression changes induced by CP‐55,940‐mediated spontaneous withdrawal.
Conclusions and Implications
The results suggest that CBD alleviates spontaneous cannabinoid withdrawal and normalizes associated gene expression changes. Future studies are needed to determine the relevance of CBD as a potential therapeutic tool for treating CUD.
Abbreviations
- AEA
anandamide
- CBD
cannabidiol
- CB receptor
cannabinoid receptor
- CUD
cannabis use disorder
- FAAH
fatty acid amide hydrolase
- NAcc
nucleus accumbens
- SMART
Spontaneous Motor Activity Recording and Tracking
- THC
Δ9‐tetrahydrocannabinol
- VTA
ventral tegmental area
Introduction
Cannabis preparations, such as hashish and marijuana, are the most commonly used illicit drugs worldwide. The data available suggest that their consumption will continue to rise in coming years, representing a serious public health problem (Volkow et al., 2014). Approximately 24% of patients initiating treatment for substance abuse are diagnosed with cannabis use disorder (CUD) (Danovitch and Gorelick, 2012). According to the latest World Drug Report (United Nations Office on Drugs and Crime, 2016), around 182.5 million people used cannabis in 2016.
To date, neither the European Medicine Agency nor the US Food and Drug Administration have approved any medications for treating CUD; however, different pharmacological approaches have been developed. These fall into two main categories: medications that attenuate symptoms of cannabis withdrawal and/or those that reduce subjective and reinforcing effects of cannabis (for a recent review, see Copeland and Pokorski, 2016). About half the patients treated for CUD report symptoms of a withdrawal syndrome. As these symptoms can serve as a negative reinforcement for relapse to cannabis use in individuals trying to abstain (Levin et al., 2010), cannabis withdrawal should be a focus of treatment. Previous clinical trials have evaluated the therapeutic usefulness of different pharmacological approaches for managing cannabis withdrawal and modulating the reinforcing effects and craving for cannabis, with inconsistent results (Danovitch and Gorelick, 2012). However, the overall clinical outcome in the treatment arms of randomized trials is poor, and fewer than 20% of participants achieve long‐term abstinence (Stephens and Roffman, 2006). In spite of the animal models developed to analyse cannabis abuse liability (Justinova et al., 2005), limited knowledge of the neurochemical mechanisms underlying CUD may contribute, at least in part, to the low efficacy of the medications evaluated to date. Therefore, it is necessary to invest effort and resources into identifying new drugs that, alone or in combination, may improve the efficacy of CUD treatment.
Recent clinical data suggest that http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150 (CBD), one of the main constituents of the Cannabis sativa plant, may hold promise as a therapeutic tool for managing CUD. Our group has recently shown that unlike Δhttp://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 (THC), CBD is devoid of rewarding psychotropic properties (Manzanares et al., 2016). Studies in animal models have shown that CBD presents anxiolytic (for a recent review, see Blessing et al., 2015), antidepressant (Zanelati et al., 2010; Schiavon et al., 2016) and antipsychotic properties (Zuardi et al., 1995; Leweke et al., 2012). Although the exact mechanisms underlying these effects remain unclear (Campos et al., 2012b), some authors have posited that CBD modulates the function of more than 65 targets in the CNS (Ibeas Bih et al., 2015), including cannabinoid receptors (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=109, the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507, the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1 (Bisogno et al., 2001; Russo et al., 2005; Ryberg et al., 2007; Thomas et al., 2007; Campos et al., 2012a), the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364 (AEA)‐hydrolysing enzyme [http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400)] and the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=216 (Carrier et al., 2006; Massi et al., 2008). However, additional studies are needed to fully determine CBD's target engagement profile.
Previous clinical results point to the therapeutic usefulness of combining CBD with THC as a ‘cannabinoid replacement therapy’ to modulate cannabinoid withdrawal (Allsop et al., 2015). Combination therapy with THC plus CBD in an oromucosal spray (nabiximols or Sativex in the United States of America or European Union, respectively) shows some interesting therapeutic benefits. Indeed, a recent clinical trial demonstrated that nabiximols suppressed cannabis withdrawal symptoms and achieved successful retention in treatment (Allsop et al., 2014). Two additional studies showed that Sativex produces a significant reduction in cannabis withdrawal score, craving and cannabis consumption levels (Trigo et al., 2016a; Trigo et al., 2016b). However, the presence of THC in nabiximols/Sativex preparations could be problematic, especially in (still unexplored) long‐term treatment, since it may be associated with THC‐related negative psychoactive effects. Thus, recent interest has turned to clinically evaluating CBD alone for managing CUD‐related problems. Crippa et al. (2013) found that CBD monotherapy led to a rapid decrease in cannabis withdrawal symptoms. Another clinical study, comparing p.o. CBD alone versus placebo, found no difference between groups for cannabis‐induced subjective effects (Haney et al., 2016). These reports join a raft of ongoing clinical trials evaluating the effects of CBD alone for CUD (Mclean Hospital, NCT03102918), cannabis dependence (University College, London, NCT02044809), cannabis withdrawal (The University of New South Wales, NCT02083874) or smoked marijuana's (5.6% THC) subjective, reinforcing, cognitive and cardiovascular effects (National Institute on Drug Abuse, NCT01844687). In the context of this increasing interest in CBD for managing CUD, studies using animal models are crucial to providing information about the therapeutic potential of CBD and the underlying neurobiological mechanisms involved.
The objective of this study is to evaluate the effects of CBD on the spontaneous withdrawal induced by the repeated administration of the potent synthetic cannabinoid receptor agonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=730 (Devane et al., 1988; Wiley et al., 1995; Oliva et al., 2003; 2004; Aracil‐Fernandez et al., 2013) in C57BL/6J mice. Motor activity (distance travelled in the open field test), withdrawal‐related somatic signs (number of rearings, groomings, rubbings and jumpings evaluated in the open field test) and the anxiety‐like response (light–dark box test) were assessed 12 h after the last administration of CP‐55,940. Furthermore, gene expression analyses by real‐time PCR were carried out to evaluate the changes induced by cannabinoid withdrawal in specific key targets involved in cannabinoid addiction and withdrawal (Romero et al., 1998; Corchero et al., 1999; Manzanares et al., 1999; Oliva et al., 2003, 2004; Lupica et al., 2004; Corchero et al., 2004a,b; Fattore et al., 2008; Aracil‐Fernandez et al., 2013), namely, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1243 in the ventral tegmental area (VTA), and the opioid http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319&familyId=50&familyType=GPCR (Oprm1), CB1 receptor (Cnr1) and CB2 receptor (Cnr2) in the nucleus accumbens (NAcc). Briefly, TH is the rate‐limiting enzyme for dopamine synthesis in the VTA, playing a pivotal role in the rewarding effects of cannabinoids. Similarly, the μ receptor in the NAcc plays a crucial role in the reinforcing actions of cannabinoid drugs, increasing the release of endogenous opioid peptides that in turn enhance the dopamine tone. Finally, CB1 and CB2 receptors directly modulate the neurobiological actions produced by the administration of cannabinoid compounds.
Methods
Mice
A total of 180 male C57BL/6J mice from Charles River (Lille, France) weighing 20–25 g were employed in this study, and were housed in groups of five per cage (40 × 25 × 22 cm) under controlled conditions (temperature, 23 ± 2°C; relative humidity, 60 ± 10%; and 12 h light/dark cycle, lights on from 08:00 to 20:00 h). One week after acclimatization to the animal room, behavioural analyses were initiated and performed by placing the home cage in the operant‐task room during the development of conditioning experiments. All studies complied with the Spanish Royal Decree 53/2013, the Spanish Law 32/2007 and the European Union Directive of the 22nd of September 2010 (2010/63/UE) regulating the care of experimental animals, and were approved by the ethics committee of Miguel Hernandez University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Drugs
Cannabinoid CB1 receptor agonist CP‐55,940 ((−)‐cis‐3‐(2‐hydroxy‐4‐(1,1,dimethyl‐heptyl)‐phenyl)‐trans‐4(3‐hydroxypropyl)cyclohexanol) obtained from Biogen (Madrid, Spain) was dissolved in ethanol : cremophor : saline (1:1:18) immediately before use and administered i.p. (0.5 mg·kg−1 every 12 h, for 7 days) as described elsewhere (Aracil‐Fernandez et al., 2013). CBD, obtained from STI Pharmaceuticals (Essex, UK), was dissolved in ethanol : cremophor : saline (1:1:18) immediately before use to obtain the required doses (5, 10 and 20 mg·kg−1) and administered i.p. during spontaneous cannabinoid withdrawal 90 min before behavioural evaluation according to plasma and brain pharmacokinetic profiles (Deiana et al., 2012).
Experimental design
Evaluation of CBD effects on CP‐55,940‐induced spontaneous cannabinoid withdrawal
In order to evaluate cannabinoid tolerance and withdrawal, the synthetic cannabinoid agonist CP‐55,940 was employed as described previously (Oliva et al., 2003; 2004; Aracil‐Fernandez et al., 2013). Forty C57BL/6J male mice were injected with CP‐55,940 (0.5 mg·kg−1 every 12 h, i.p.) and 10 mice with vehicle (ethanol : cremophor : saline; 1:1:18) for 7 days (schematic diagram displayed in Figure 1A). Changes in rectal temperature and motor activity were measured during tolerance development (days 1, 3 and 7). On the day of withdrawal and following the last cannabinoid administration, CP‐55,940‐treated mice were randomly allocated to four experimental groups to be administered CBD (5, 10 and 20 mg·kg−1, i.p., 10 mice per dose) or its corresponding vehicle, 90 min before the evaluation of motor activity and behavioural signs associated with abstinence (number of rearings, groomings, rubbings and jumpings) in a 15 min session. At 150 min after administration of the study drug (CBD or vehicle), the brains of these mice were collected for the analysis of alterations in gene transcription. An additional set of 50 C57BL/6J male mice was evaluated during the abstinence period to study the effects of CBD treatment on anxiety‐like behaviour induced by cannabinoid withdrawal, following the same randomization procedure to assign animals to the different treatment groups. All the behavioural paradigms (open field test to evaluate motor activity and somatic signs and light–dark box test to evaluate anxiety‐like level) were made under blind conditions.
Figure 1.
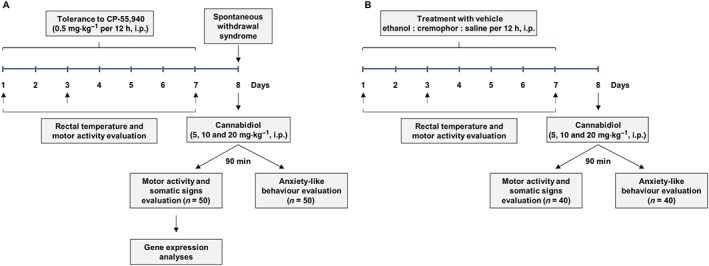
Experimental designs to induce and evaluate the spontaneous cannabinoid withdrawal (A) and analyse the effects of CBD administration in vehicle‐treated animals (B).
Evaluation of CBD effects on vehicle‐treated animals
Forty C57BL/6J male mice were injected with vehicle (ethanol : cremophor : saline; 1:1:18; twice a day every 12 h, i.p.) for 7 days (schematic diagram displayed in Figure 1B) in order to study the effects of CBD alone. Changes in rectal temperature and motor activity were measured during treatment with vehicle (days 1, 3 and 7). On the day after the last vehicle administration, mice were randomly allocated to four experimental groups to be administered CBD (5, 10 and 20 mg·kg−1, i.p., 10 mice per dose) or its corresponding vehicle, 90 min before the evaluation of motor activity and behavioural signs associated with abstinence (number of rearings, groomings, rubbings and jumpings) in a 15 min session. An additional set of 40 C57BL/6J male mice was evaluated after the last vehicle administration to study the effects of CBD treatment on anxiety‐like behaviour, following the same randomization procedure to assign animals to the different treatment groups. All the behavioural paradigms (open field test to evaluate motor activity and somatic signs and light–dark box test to evaluate anxiety‐like level) were made under blind conditions.
Assessment of tolerance to cannabinoid administration
Motor behaviour – open field test
On day 0, under baseline conditions and 60 min after the administration of CP‐55,940 or vehicle on days 1, 3 and 7, the mice were placed into individual methacrylate boxes (25 × 25 × 25 cm), and their motor responses were evaluated according to distance travelled for 15 min. The assessment of motor activity was carried out by using the Spontaneous Motor Activity Recording and Tracking (SMART) programme by Panlab (Barcelona, Spain).
Rectal temperature
Rectal temperature was measured in each mouse using a lubricated digital thermistor probe (Panlab), inserted 1 cm into the rectum for 30 s. Rectal temperature was determined 30 min before and after each morning injection of CP‐55,940 or vehicle during the tolerance period on days 1, 3 and 7.
Behavioural assessment after cannabinoid withdrawal
Behaviour in CP‐55,940‐induced spontaneous cannabinoid withdrawal and vehicle‐treated animals was assessed by measuring motor activity; the number of rearings, groomings, rubbings, jumpings; and the anxiety‐like behaviour on the day after the cessation of cannabinoid or vehicle treatment.
Motor activity and somatic expression
Twelve hours after cessation of treatment with CP‐55,940 or vehicle, mice were placed into individual methacrylate boxes (25 × 25 × 25 cm). Ninety minutes after CBD (5, 10 and 20 mg·kg−1) or vehicle administration, mice behaviour was videotaped for 15 min, and the somatic signs associated with abstinence (number of rearings, groomings, rubbings and jumpings) were subsequently analysed from the recording. At the same time, motor responses were also evaluated by measuring the distance travelled by the mice for 15 min with the SMART programme (Panlab).
Light–dark box test
To evaluate anxiety‐like behaviour associated with cannabinoid withdrawal, 12 h after cessation of treatment with CP‐55,940 or vehicle, mice were individually tested for 5 min in the light–dark box paradigm, 90 min after the administration of CBD (5, 10 and 20 mg·kg−1) or its corresponding vehicle. At the beginning of the session, the mice were placed in the tunnel facing the dark side. The number of transitions and the time spent on the light side were measured in each session.
Gene expression studies by real‐time PCR
Relative gene expression analyses of TH in the VTA, and Oprm1, Cnr1 and Cnr2 in the NAcc, were carried out in vehicle‐treated and CP‐55,940‐treated C57BL/6J mice employed to evaluate the effects of CBD on motor activity and somatic signs during CP‐55,940‐induced spontaneous cannabinoid withdrawal (see Figure 1A). Briefly, mice were killed by cervical dislocation 150 min after CBD administration on day 8. Brains were removed from the skull and frozen over dry ice. Coronal sections (500 μm) containing the regions of interest were cut in a cryostat (−10°C) according to the Paxinos and Franklin atlas (Paxinos and Franklin, 2001), mounted onto slides and stored at −80°C. Sections were microdissected following the method described by Palkovits (Palkovits, 1983). Total RNA was obtained from brain micropunches by treatment with TRI Extraction Reagent (Applied Biosystems, Madrid, Spain). Reverse transcription to cDNA was carried out following the manufacturer's instructions (Applied Biosystems). The relative abundance of TH (Mm00447546_m1), Oprm1 (Mm01188089_m1), Cnr1 (Mm00432621_s1) and Cnr2 (Mm00438286_m1) gene expression was quantified in a StepOne Plus Sequence Detector System (Life Technologies, Madrid, Spain). Each assay was undertaken in technical duplicate to ensure the reliability of single values and the average calculated for data analyses. All reagents were obtained from Life Technologies according to the manufacturer's protocols. The reference gene used was 18S rRNA (Mm03928990_g1). All primer–probe combinations were optimized and validated for relative quantification of gene expression. Data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene expression was determined using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical analyses
Statistical analyses were performed using one‐ and two‐way ANOVA with repeated measures (RM) when several time points are present, followed by the Student–Newman–Keul's test when comparing different experimental groups. Differences were considered significant if the probability of error was less than 5%. Data are presented as mean ± SEM. Sigmaplot 11 software (Systat software Inc., Chicago, IL, USA) was used for all statistical analyses. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Table 1 presents all the statistical data included in the results section. P values < 0.05 were considered to indicate statistical significance.
Table 1.
Details of the statistical results from the different experimental procedures performed in the present study
| Experiment | Statistical test | Parameter | Factors | d.f. | F | P‐value |
|---|---|---|---|---|---|---|
| Evaluation of tolerance development to CP‐55,940 administration | Two‐way RM ANOVA | Motor activity | Treatment | 1, 149 | 21.443 | <0.001 |
| Day | 2, 149 | 4.231 | <0.016 | |||
| Treatment × day | 2, 149 | 25.210 | <0.001 | |||
| Rectal temperature | Treatment | 1, 149 | 175.968 | <0.001 | ||
| Day | 2, 149 | 75.524 | <0.001 | |||
| Treatment × day | 2, 149 | 70.372 | <0.001 | |||
| Evaluation of CBD effects on spontaneous cannabinoid withdrawal behaviour | One‐way ANOVA | Motor activity | Distance travelled (cm) | 4, 49 | 8.116 | <0.001 |
| Somatic signs | Number of rearings | 4, 49 | 6.623 | <0.001 | ||
| Number of groomings | 4, 49 | 10.055 | <0.001 | |||
| Number of rubbings | 4, 49 | 5.684 | <0.001 | |||
| Number of jumpings | 4, 49 | 10.313 | <0.001 | |||
| Anxiety‐like behaviour | Time spent in light box (s) | 4, 49 | 16.157 | <0.001 | ||
| Number of transitions | 4, 49 | 11.982 | <0.001 | |||
| Evaluation of CBD effects on gene expression changes induced by spontaneous cannabinoid withdrawal | One‐way ANOVA | Relative gene expression (real‐time PCR) | TH | 4, 49 | 8.797 | <0.001 |
| Oprm1 | 4, 49 | 22.859 | <0.001 | |||
| Cnr1 | 4, 49 | 9.298 | <0.001 | |||
| Cnr2 | 4, 49 | 4.141 | 0.007 | |||
| Evaluation of motor activity and rectal temperature in vehicle‐treated animals | One‐way ANOVA | Motor activity | Distance travelled (cm) | 2, 119 | 18.844 | <0.001 |
| Rectal temperature | Temperature change (°C) | 2, 119 | 1.586 | 0.209 | ||
| Evaluation of CBD effects on motor activity and somatic signs in vehicle‐treated animals | One‐way ANOVA | Motor activity | Distance travelled (cm) | 3, 39 | 0.716 | 0.551 |
| Somatic signs | Number of rearings | 3, 39 | 0.253 | 0.859 | ||
| Number of groomings | 3, 39 | 0.232 | 0.874 | |||
| Number of rubbings | 3, 39 | 0.148 | 0.930 | |||
| Anxiety‐like behaviour | Time spent in light box (s) | 3, 39 | 4.988 | 0.007 | ||
| Number of transitions | 3, 39 | 1.484 | 0.240 |
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Results
Development of tolerance induced by the administration of CP‐55,940
The development of tolerance to cannabinoid administration was evaluated by measuring motor behaviour and rectal temperature. Two‐way RM ANOVA revealed decreased motor activity in the CP‐55,940‐treated group on day 1 compared with the other days and with the vehicle‐treated group only on day 1 [Figure 2A; treatment (P < 0.001), day (P = 0.016) and treatment × day interaction (P < 0.001)]. Two‐way ANOVA showed that CP‐55,940 administration significantly decreased rectal temperature in comparison with the vehicle‐treated group on days 1 and 3, reaching similar temperatures to control mice on day 7 [Figure 2B; treatment (P < 0.001), day (P < 0.001) and treatment × day interaction (P < 0.001)].
Figure 2.
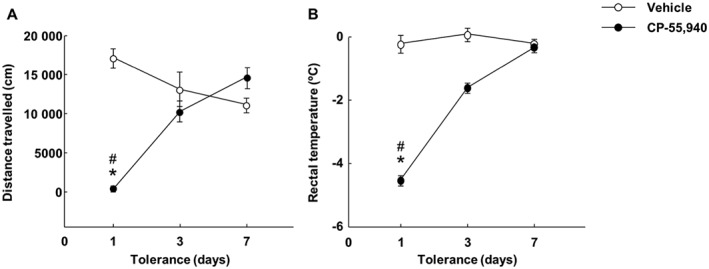
Development of tolerance after repeated administration of CP‐55,940. The mice were injected with CP‐55,940 (0.5 mg·kg−1 every 12 h, i.p.; for 7 days) or with its vehicle. (A) The effects of CP‐55,940 administration on motor behaviour. On days 1, 3 and 7, motor activity was measured for 15 min (60 min after the morning CP‐55,940 administration) to evaluate tolerance due to cannabinoid treatment. Symbols represent the means and vertical lines ±SEM for distance travelled by mice in the open field test. *Values from CP‐55,940‐treated mice that are significantly different (P < 0.05) from vehicle‐treated mice. #Values from CP‐55,940‐treated mice on day 1 that are significantly different (P < 0.05) from CP‐55,940‐treated mice treated on days 3 and 7 (two‐way RM ANOVA followed by Student–Newman–Keul's test). (B) The changes in rectal temperature induced by CP‐55,940 treatment. On days 1, 3 and 7, rectal temperature was measured (30 min before and after the morning CP‐55,940 administration) to evaluate tolerance. Symbols represent the means and vertical lines ±SEM of differences in rectal temperature. *Values from CP‐55,940‐treated mice that are significantly different (P < 0.05) from vehicle‐treated mice. #Values from CP‐55,940‐treated mice on day 1 that are significantly different (P < 0.05) from CP‐55,940‐treated mice on days 3 and 7 (two‐way RM ANOVA followed by Student–Newman–Keul's test).
Effects of CBD on increased motor activity and somatic signs induced by spontaneous cannabinoid withdrawal
The one‐way ANOVA showed that CP‐55,940 treatment cessation significantly increased motor activity and the number of rearings, rubbings and jumpings compared with vehicle‐treated mice [Figure 3A, motor activity (P < 0.001); Figure 3B, number of rearings (P < 0.001); Figure 3D, number of rubbings (P < 0.001); and Figure 3E, number of jumpings (P < 0.001)]. The administration of CBD fully blocked these parameters, which reached values similar to those found in the control group. On the other hand, the number of groomings after cannabinoid cessation decreased significantly compared with the control group. One‐way ANOVA indicated that the CP‐55,940 + CBD‐treated group showed a normalization effect reaching a significant difference compared with the CP‐55,940 + vehicle‐treated group with the highest CBD dose (Figure 3C, number of groomings, P < 0.001).
Figure 3.
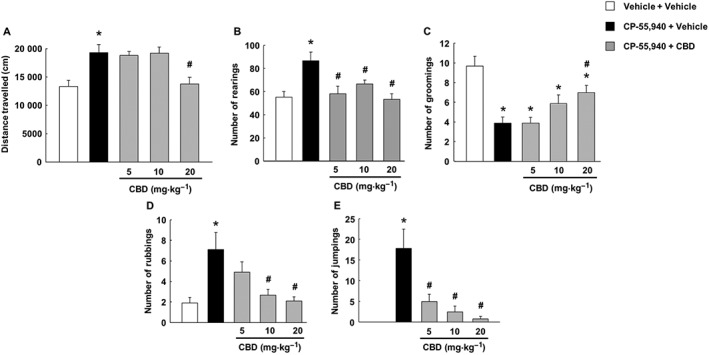
Assessment of spontaneous cannabinoid withdrawal and CBD actions on motor activity and behavioural signs of abstinence (number of rearings, groomings, rubbings and jumpings). Mice were injected with CP‐55,940 (0.5 mg·kg−1 every 12 h, i.p.) or vehicle (ethanol : cremophor : saline; 1:1:18) for 7 days. CBD (5, 10 and 20 mg·kg−1, i.p.) or vehicle (ethanol : cremophor : saline; 1:1:18) was administered approximately 12 h after the last CP‐55,940 administration. Mice received the CBD dose, and 90 min later, motor activity and behavioural signs were evaluated in 15 min sessions. Columns represent the means and vertical lines ±SEM of distance travelled (cm) by mice in the open field test (A) and the number of rearings (B), groomings (C), rubbings (D) and jumpings (E). *Values from CP‐55,940‐treated mice that are significantly different (P < 0.05) from vehicle + vehicle‐treated mice. #Values from mice treated with CP‐55,940 + CBD that are significantly different (P < 0.05) from CP‐55,940 + vehicle‐treated mice (one‐way ANOVA followed by Student–Newman–Keul's test).
Effects of CBD on anxiogenic‐like behaviour induced by spontaneous cannabinoid withdrawal
After CP‐55,940 treatment, the one‐way ANOVA revealed a significant decrease in the time spent in the light box (Figure 4A, P < 0.001) and the number of transitions (Figure 4B, P < 0.001) in the CP‐55,940 + vehicle‐treated group compared with the control group. The administration of CBD blocked, at all doses, the decrease in the time spent in the lighted area, which reached values similar to those found in the control group, without affecting the number of transitions compared with the CP‐55,940 + vehicle‐treated group.
Figure 4.
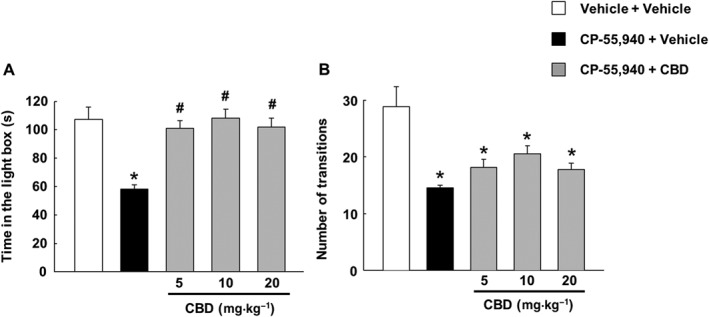
Assessment of anxiety‐like behaviour associated with spontaneous cannabinoid withdrawal and subsequent treatment with CBD. Mice were injected with CP‐55,940 (0.5 mg·kg−1 every12 h, i.p.) or vehicle (ethanol : cremophor : saline; 1:1:18) for 7 days. CBD (5, 10 and 20 mg·kg−1, i.p.) or its vehicle (ethanol : cremophor : saline; 1:1:18) was administered 12 h after the last administration of CP‐55,940. Mice received the CBD dose, and 90 min later, they were exposed to the light–dark box paradigm for 5 min. (A) The evaluation of the time in the light box and (B) the evaluation of the number of transitions. Columns represent the means and vertical lines ±SEM of time (s) on the light side and number of transitions. *Values from CP‐55,940‐treated mice that are significantly different (P < 0.05) from the control group. #Values from CP‐55,940 + CBD‐treated mice that are significantly different (P < 0.05) from the CP‐55,940 + vehicle‐treated mice (one‐way ANOVA followed by Student–Newman–Keul's test).
Effects of CBD on changes in the TH, Oprm1, Cnr1 and Cnr2 gene expression induced by spontaneous cannabinoid withdrawal
Changes in TH gene expression, and in Oprm1, Cnr1 and Cnr2 gene expressions, were measured in the VTA and in the NAcc respectively. The results showed that cessation of CP‐55,940 administration reduced TH (Figure 5A, one‐way ANOVA, P < 0.001) and Cnr2 gene expression (Figure 5D, one‐way ANOVA, P = 0.007), whereas it increased both Oprm1 (Figure 5B, one‐way ANOVA, P < 0.001) and Cnr1 gene expressions (Figure 5C, one‐way ANOVA, P < 0.001). The administration of CBD blocked the effects of cannabinoid withdrawal on TH, Oprm1, Cnr1 and Cnr2 gene expression.
Figure 5.
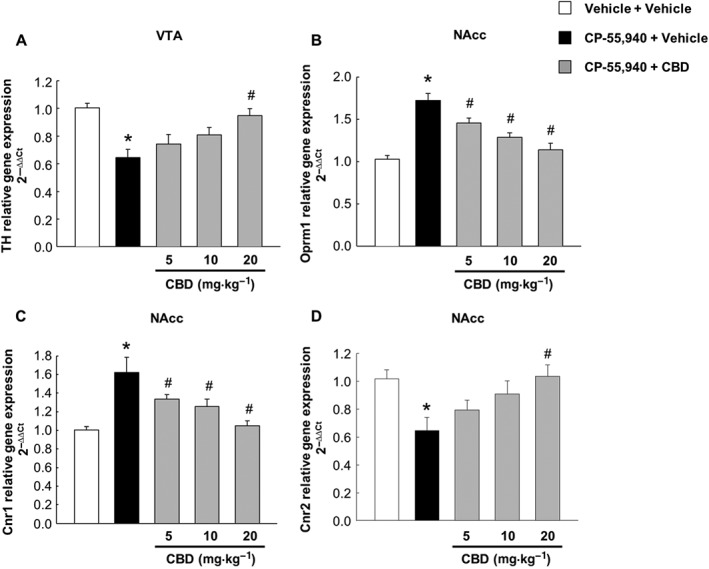
Gene expression changes induced by spontaneous cannabinoid withdrawal and subsequent CBD treatment. Evaluation of TH gene expression in the VTA (A) and of Oprm1 (B), Cnr1 (C) and Cnr2 (D) in the NAcc. Columns represent the means and vertical lines ±SEM of 2−ΔΔCt. *Values from CP‐55,940‐treated mice that are significantly different (P < 0.05) from vehicle‐treated mice. #Values from CP‐55,940 + CBD‐treated mice that are significantly different (P < 0.05) from CP‐55,940 + vehicle‐treated mice (one‐way ANOVA followed by Student–Newman–Keul's test).
Evaluation of motor activity and rectal temperature during 7 days of vehicle administration
Distance travelled and rectal temperature variation (30 min before and after administration) was evaluated in the animals treated with vehicle. Motor activity progressively decreased from day 1 to day 7 in the open field (Figure 6A, one‐way ANOVA, P < 0.001) reflecting a habituation effect similar to that observed in the vehicle‐treated animals represented in Figure 2. With regard to rectal temperature, the vehicle group did not show any change between the evaluation days (Figure 6B, one‐way ANOVA, P = 0.209).
Figure 6.
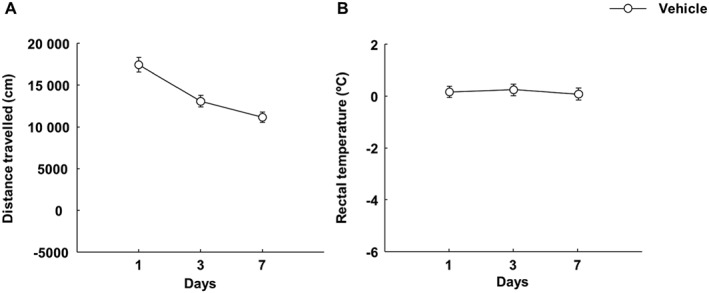
Assessment of motor activity and rectal temperature variations in mice injected i.p. with vehicle (ethanol : cremophor : saline; 1:1:18; every 12 h) for 7 days. (A) The effects of vehicle administration on motor behaviour. On days 1, 3 and 7, motor activity was measured for 15 min (60 min after the morning vehicle administration). Symbols represent the means and vertical lines ±SEM of distance travelled by mice in the open field test. (B) The changes in rectal temperature induced by vehicle treatment. On days 1, 3 and 7, rectal temperature was measured (30 min before and after the morning vehicle administration). Symbols represent the means and vertical lines ±SEM of differences in rectal temperature.
Effects of CBD on motor activity and somatic signs in vehicle‐treated animals
CBD administration did not induce changes in the somatic signs displayed by the vehicle‐treated animals (as assessed by one‐way ANOVA) suggesting that the normalization effect achieved in CP‐55,940‐treated animals was specific [Figure 7A, motor activity (P = 0.551); Figure 7B, number of rearings (P = 0.859); Figure 7C, number of groomings (P = 0.874); and Figure 7D, number of rubbings (P = 0.930)]. Figure 7 does not present the number of jumpings as vehicle‐treated animals did not display this behaviour.
Figure 7.
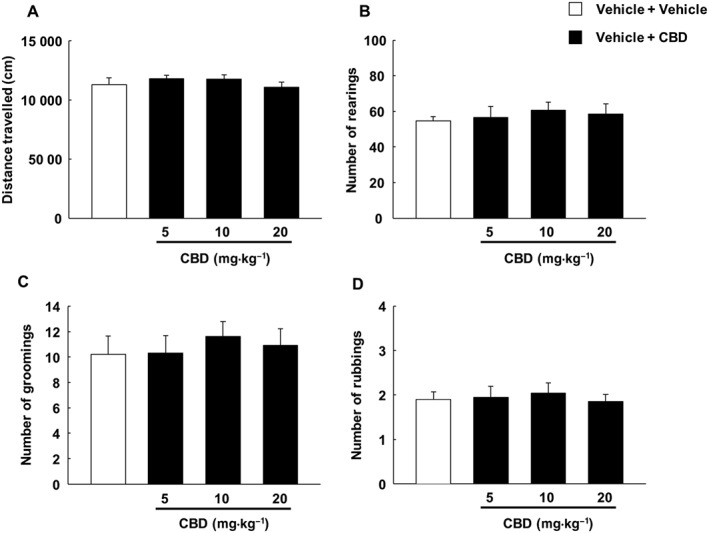
Assessment of effects of CBD on motor activity and behavioural signs of abstinence (number of rearings, groomings and rubbings) in mice injected i.p. with vehicle (ethanol : cremophor : saline; 1:1:18; every 12 h) for 7 days. CBD (5, 10 and 20 mg·kg−1, i.p.) or vehicle (ethanol : cremophor : saline; 1:1:18) was administered approximately 12 h after the last vehicle administration. Mice received the CBD dose, and 90 min later, motor activity and behavioural signs were evaluated in 15 min sessions. Columns represent the means and vertical lines ±SEM of distance travelled (cm) by mice in the open field test (A) and the number of rearings (B), groomings (C) and rubbings (D). The number of jumpings is not displayed as the vehicle‐treated animals did not show this behavioural sign.
Effects of CBD on anxiety‐like behaviour in vehicle‐treated animals
Anxiety‐like behaviour in vehicle‐treated animals was reduced significantly after the administration of CBD (10 and 20 mg·kg−1), increasing the latency time in the light box (Figure 8A, P = 0.007), whereas the number of transitions was similar between the different treatment groups (Figure 8B, P = 0.240).
Figure 8.
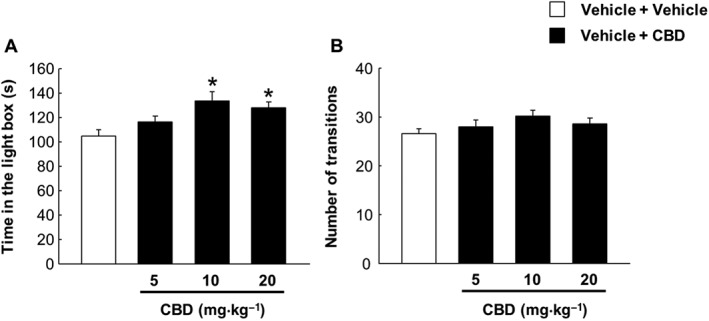
Effects of CBD on anxiety‐like behaviour in mice injected i.p. with vehicle (ethanol : cremophor : saline; 1:1:18; every 12 h) for 7 days. CBD (5, 10 and 20 mg·kg−1, i.p.) or its vehicle (ethanol : cremophor : saline; 1:1:18) was administered approximately 12 h after the last vehicle administration. Mice received the CBD dose, and 90 min later, they were exposed to the light–dark box paradigm for 5 min. (A) The evaluation of the time in the light box and (B) the evaluation of the number of transitions. Columns represent the means and vertical lines ±SEM of time (s) in the light side and number of transitions. *Values from vehicle + CBD‐treated mice that are significantly different (P < 0.05) from the control group (vehicle + vehicle) (one‐way ANOVA followed by Student–Newman–Keul's test).
Discussion
The results of this study suggest that CBD is useful for alleviating the motor symptoms and anxiety‐like behaviours associated with spontaneous withdrawal of the cannabinoid receptor agonist CP‐55,940, with several observations supporting this assumption: (i) after cessation of treatment with CP‐55,940, CBD normalized the increase in the distance travelled and the number of rearings, rubbings and jumpings, as well as the reduction in the number of groomings; (ii) mice receiving CBD showed greatly reduced anxiety‐like behaviour associated with the cannabinoid withdrawal compared with controls; (iii) the administration of CBD also blocked the reduction in TH and Cnr2 gene expression in the VTA and NAcc, respectively, and the increase in Oprm1 and Cnr1 gene expression in the NAcc, induced by spontaneous cannabinoid withdrawal; and (iv) CBD did not modify the motor activity or the somatic signs (number of rearings, rubbings and groomings) displayed by non‐abstinent mice but induced an anxiolytic‐like effect in vehicle‐treated animals.
The reduction in motor activity and rectal temperature found on day 1 after CP‐55,940 administration returned to normal values on days 3 and 7 of treatment, respectively, indicating the development of tolerance. Subsequently, treatment with CP‐55,940 was abruptly discontinued to generate a spontaneous withdrawal (without cannabinoid antagonist administration). This was characterized by a variety of behavioural manifestations, such as an increase in distance travelled, number of rearings, rubbings and jumpings, and anxiety‐like behaviour, together with a decrease in the number of groomings. These results are in agreement with previous findings from our laboratory employing the same model of spontaneous cannabinoid withdrawal (Oliva et al., 2003; 2004; Aracil‐Fernandez et al., 2013).
The administration of CBD significantly affected the motor activity observed in mice during the spontaneous cannabinoid withdrawal, although this effect was only reached at the highest dose (20 mg·kg−1). In addition, all CBD doses tested (5, 10 and 20 mg·kg−1) blocked the increase in the number of rearings and reduced the significant increase in the number of rubbings and jumpings. The number of groomings decreased considerably during cannabinoid withdrawal, as described elsewhere (Cook et al., 1998), but the highest dose (20 mg·kg−1) of CBD administered led to a significant increase in this behaviour. Interestingly, the effects of CBD on motor activity and somatic signs were specific to the CP.55,940‐induced spontaneous withdrawal since its administration to vehicle‐treated mice (animals not developing spontaneous cannabinoid withdrawal) did not induce any significant behavioural change. Moreover, mice experiencing cannabinoid withdrawal presented a high degree of anxiety with a significant reduction in the time spent in the lighted box. All doses of CBD completely abolished this anxiogenic‐like effect without affecting the number of transitions compared with the CP‐55,940 + vehicle‐treatment group. In addition, CBD also induced an anxiolytic‐like effect in vehicle‐treated animals without modifying the number of transitions between the different treatment groups. The literature contains numerous reports supporting an anxiolytic effect for CBD; for a recent review, see Blessing et al. (2015).
The results obtained in the present study suggest that under these experimental conditions, CBD significantly alleviates the most prominent behavioural symptoms associated with CP‐55,940‐induced spontaneous cannabinoid withdrawal. To our knowledge, this is the first study that has evaluated the effect of CBD on spontaneous cannabinoid withdrawal in an animal model and explored some of the underlying neurobiological mechanisms potentially involved. Although there is currently little clinical evidence for this treatment approach and especially for the effects of CBD alone, our results are consistent with some existing findings, such as those reported by Crippa et al. (2013). Therefore, additional translational studies combining both clinical and basic perspectives are warranted to clarify the therapeutic potential of CBD for cannabinoid withdrawal symptoms. This experimental approximation will be crucial to the development of future pharmacological strategies for managing CUD at the clinical level.
Nowadays, it is well known that cannabis withdrawal – similar to withdrawal from other drugs of abuse – decreases mesolimbic dopamine neuronal activity (Oleson and Cheer, 2012). Indeed, cessation of THC treatment produced a reduction in the spontaneous firing rate of dopamine neurons in VTA similar to that observed with other addictive drugs (Diana et al., 1998). Likewise, our group's present and past research clearly demonstrates that abrupt cessation of CP‐55,940 treatment significantly decreases TH gene expression in VTA neurons (Oliva et al., 2003; Aracil‐Fernandez et al., 2013). Interestingly, CBD administration normalized this alteration, increasing the mRNA levels of TH in the VTA. In line with this result, a recent study from our laboratory also revealed that CBD modulates TH gene expression in animals exposed to EtOH under different behavioural paradigms (Viudez‐Martinez et al., 2018). The mechanisms that could be involved in these phenomena are still unknown, although several hypotheses have recently emerged regarding the effects of CBD on the mesolimbic dopamine system (Renard et al., 2017). Indeed, one of the well‐described CBD's mechanisms of action is the inhibition of FAAH, which in turn has been associated with TH modulation (Bosier et al., 2013). Therefore, the supposed regulation of dopamine synthesis by means of CBD‐mediated effects on TH gene expression could be involved in the alleviation of the effects of cannabinoid withdrawal.
There is evidence supporting the interaction between the endogenous opioid and cannabinoid systems (Corchero et al., 2004a). Previous results demonstrated that cannabinoid administration increases the release of endogenous opioid peptides and opioid‐related gene expression, mechanisms closely involved in the regulation of cannabinoid reinforcing actions (Manzanares et al., 1999; Corchero et al., 2004b). Indeed, our results indicate that spontaneous withdrawal induced by interruption of cannabinoid receptor agonist administration significantly increased Oprm1 gene expression in the NAcc, and this effect was completely normalized by the administration of CBD. Interestingly, Kathmann et al. (2006) described the CBD‐mediated allosteric modulation of the μ receptor by means of kinetic binding studies with [3H]‐DAMGO, although the observed effect only occurred at very high concentrations and cannot be expected to contribute to its in vivo action. In addition, CBD significantly down‐regulated Oprm1 in the NAcc, with this effect being accompanied by a reduction in EtOH consumption (Viudez‐Martinez et al., 2018). Thus, it is reasonable to suggest that the CBD‐mediated regulation of the μ receptor function could be involved in alleviating cannabinoid withdrawal symptoms, as well as those of opioid withdrawal, as proposed elsewhere (Hine et al., 1975a,b; Bhargava, 1976).
In our study, as in previous reports (Oliva et al., 2003; 2004; Aracil‐Fernandez et al., 2013), Cnr1 gene expression was significantly up‐regulated in the NAcc. Different authors have attributed this increase to a compensatory neuroadaptative response to down‐regulation of cannabinoid receptors found after repeated treatment with a cannabinoid receptor agonist (Sim et al., 1996; Romero et al., 1998). CBD reduced the Cnr1 up‐regulation induced by spontaneous cannabinoid withdrawal. This result is also in agreement with CBD‐induced reduction associated with a decrease in EtOH intake in C57BL/6J mice (Viudez‐Martinez et al., 2018). Previous studies suggested that CBD acts as a non‐competitive allosteric modulator of the cannabinoid CB1 receptor through the alteration of AEA hydrolysis by inhibiting its catabolic enzyme, FAAH (Bisogno et al., 2001; Laprairie et al., 2015). Based on this assumption, it is tempting to hypothesize that the modification of the AEA concentrations could be related, at least in part, to the neurochemical changes induced by CBD and the modulation of the cannabinoid withdrawal syndrome. However, additional experiments are necessary to shed light on this matter.
Finally, previous studies have shown that the cannabinoid CB2 receptor is involved in regulating addictive behaviours, suggesting that this target plays a pivotal role in modulating the reinforcing effects of cocaine, nicotine and alcohol (Aracil‐Fernandez et al., 2012; Navarrete et al., 2013; Ortega‐Alvaro et al., 2015). However, there is very little information regarding the involvement of CB2 receptors in cannabis addiction, or specifically in cannabis withdrawal. The real‐time PCR analyses from the present study show for the first time that spontaneous cannabinoid withdrawal is linked to a significant Cnr2 down‐regulation in the NAcc, although the underlying mechanisms have yet to be elucidated. As CBD up‐regulates Cnr2 gene expression (Viudez‐Martinez et al., 2018), this normalization effect could be related to the improvement in cannabinoid withdrawal behavioural disturbances induced by the administration of CBD. There is controversy with regard to the pharmacological effect of CBD on CB2 receptors, but a recent report points out that CBD acts as an allosteric modulator (Martinez‐Pinilla et al., 2017). Future studies are needed to better understand the interaction between CBD and CB2 receptors.
In conclusion, the results of the present study provide unequivocal evidence for the efficacy of CBD to reduce the behavioural disturbances associated with CP‐55,940‐induced spontaneous cannabinoid withdrawal. Furthermore, the gene expression analyses of targets closely involved in the cannabinoid‐related addictive actions and withdrawal (TH, Oprm1, Cnr1 and Cnr2) provide valuable information about the neurochemical processes that could be involved, at least in part, in the CBD‐mediated regulation of cannabinoid withdrawal. Further studies are needed to evaluate the potential therapeutic actions of CBD for the clinical management of CUD and to elucidate the precise underlying neurobiological mechanisms involved.
Author contributions
All named authors made an active contribution to the conception, design, performance and statistical analysis of the results. A.A.‐F. and F.N. carried out the behavioural analyses. A.A.‐F. and F.N. performed the real‐time PCR analyses. F.N. wrote the first draft of the manuscript. F.N. and J.M. critically reviewed the content, validated the accuracy of the data and approved the final version for publication.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This research was supported by the ‘Instituto de Salud Carlos III’ (RTA, RD12/0028/0019 and RD16/0017/0014, Fondos FEDER), ‘Plan Nacional Sobre Drogas’ (PNSD 2016/016) and ‘Ministerio de Economía y Competitividad’ (FIS, PI14/00438).
Navarrete, F. , Aracil‐Fernández, A. , and Manzanares, J. (2018) Cannabidiol regulates behavioural alterations and gene expression changes induced by spontaneous cannabinoid withdrawal. British Journal of Pharmacology, 175: 2676–2688. doi: 10.1111/bph.14226.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C et al (2014). Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiat 71: 281–291. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Lintzeris N, Copeland J, Dunlop A, McGregor IS (2015). Cannabinoid replacement therapy (CRT): nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Ther 97: 571–574. [DOI] [PubMed] [Google Scholar]
- Aracil‐Fernandez A, Almela P, Manzanares J (2013). Pregabalin and topiramate regulate behavioural and brain gene transcription changes induced by spontaneous cannabinoid withdrawal in mice. Addict Biol 18: 252–262. [DOI] [PubMed] [Google Scholar]
- Aracil‐Fernandez A, Trigo JM, Garcia‐Gutierrez MS, Ortega‐Alvaro A, Ternianov A, Navarro D et al (2012). Decreased cocaine motor sensitization and self‐administration in mice overexpressing cannabinoid CB2 receptors. Neuropsychopharmacology 37: 1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN (1976). Effect of some cannabinoids on naloxone‐precipitated abstinence in morphine‐dependent mice. Psychopharmacology (Berl) 49: 267–270. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I et al (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Lambert DM (2013). The FAAH inhibitor URB597 efficiently reduces tyrosine hydroxylase expression through CB1‐ and FAAH‐independent mechanisms. Br J Pharmacol 169: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, Guimaraes FS (2012a). Cannabidiol blocks long‐lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res 46: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS (2012b). Multiple mechanisms involved in the large‐spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367: 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 103: 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR (1998). CB1 receptor antagonist precipitates withdrawal in mice exposed to Δ9‐tetrahydrocannabinol. J Pharmacol Exp Ther 285: 1150–1156. [PubMed] [Google Scholar]
- Copeland J, Pokorski I (2016). Progress toward pharmacotherapies for cannabis‐use disorder: an evidence‐based review. Subst Abuse Rehabil 7: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchero J, Manzanares J, Fuentes JA (2004a). Cannabinoid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol 16: 159–172. [DOI] [PubMed] [Google Scholar]
- Corchero J, Oliva JM, Garcia‐Lecumberri C, Martin S, Ambrosio E, Manzanares J (2004b). Repeated administration with δ9‐tetrahydrocannabinol regulates μ‐opioid receptor density in the rat brain. J Psychopharmacol 18: 54–58. [DOI] [PubMed] [Google Scholar]
- Corchero J, Romero J, Berrendero F, Fernandez‐Ruiz J, Ramos JA, Fuentes JA et al (1999). Time‐dependent differences of repeated administration with Δ9‐tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G‐protein activation by μ‐opioid and CB1‐cannabinoid receptors in the caudate–putamen. Brain Res Mol Brain Res 67: 148–157. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Hallak JE, Machado‐de‐Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH et al (2013). Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther 38: 162–164. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovitch I, Gorelick DA (2012). State of the art treatments for cannabis dependence. Psychiatr Clin North Am 35: 309–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S et al (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9‐tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology (Berl) 219: 859–873. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988). Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: 605–613. [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL (1998). Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A 95: 10269–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Spano MS, Pistis M, Fratta W (2008). Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol 286 (1‐2 Suppl 1): S97–S107. [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G et al (2016). Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 41: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine B, Torrelio M, Gershon S (1975a). Differential effect of cannabinol and cannabidiol on THC‐induced responses during abstinence in morphine‐dependent rats. Res Commun Chem Pathol Pharmacol 12: 185–188. [PubMed] [Google Scholar]
- Hine B, Torrelio M, Gershon S (1975b). Interactions between cannabidiol and Δ9‐THC during abstinence in morphine‐dependent rats. Life Sci 17: 851–857. [DOI] [PubMed] [Google Scholar]
- Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12: 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G (2005). Self‐administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 81: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Trankle C, Schlicker E (2006). Cannabidiol is an allosteric modulator at mu‐ and delta‐opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 372: 354–361. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Denovan‐Wright EM (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172: 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL et al (2010). Cannabis withdrawal symptoms in non‐treatment‐seeking adult cannabis smokers. Drug Alcohol Depend 111: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C et al (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔC T method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF (2004). Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol 143: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez‐Ruiz JJ, Ramos JA, Fuentes JA (1999). Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci 20: 287–294. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Medrano‐Relinque J, Viudez‐Martinez A, Navarron CM, Aracil‐Fernandez A, Navarrete F , et al (2016). Cannabidiol a drug lacking reinforcing activity. 77.02/AAA18. In: Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2016. Online.
- Martinez‐Pinilla E, Varani K, Reyes‐Resina I, Angelats E, Vincenzi F, Ferreiro‐Vera C et al (2017). Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol 8: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E et al (2008). 5‐Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non‐psychoactive cannabinoid. J Neurochem 104: 1091–1100. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete F, Rodriguez‐Arias M, Martin‐Garcia E, Navarro D, Garcia‐Gutierrez MS, Aguilar MA et al (2013). Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology 38: 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF (2012). A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2: pii: a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, Manzanares J (2003). Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. J Neurochem 85: 94–104. [DOI] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, Manzanares J (2004). Spontaneous cannabinoid withdrawal produces a differential time‐related responsiveness in cannabinoid CB1 receptor gene expression in the mouse brain. J Psychopharmacol 18: 59–65. [DOI] [PubMed] [Google Scholar]
- Ortega‐Alvaro A, Ternianov A, Aracil‐Fernandez A, Navarrete F, Garcia‐Gutierrez MS, Manzanares J (2015). Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol 20: 43–55. [DOI] [PubMed] [Google Scholar]
- Palkovits M (1983). Punch sampling biopsy technique. Methods Enzymol 103: 368–376. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin (2001). The Mouse Brain in Stereotaxic Coordinates. Academic Press: Harcourt Science and Technology Company: New York. [Google Scholar]
- Renard J, Norris C, Rushlow W, Laviolette SR (2017). Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev 75: 157–165. [DOI] [PubMed] [Google Scholar]
- Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA et al (1998). Time‐course of the cannabinoid receptor down‐regulation in the adult rat brain caused by repeated exposure to Δ9‐tetrahydrocannabinol. Synapse 30: 298–308. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK (2005). Agonistic properties of cannabidiol at 5‐HT1a receptors. Neurochem Res 30: 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J et al (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152: 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon AP, Bonato JM, Milani H, Guimaraes FS, Weffort de Oliveira RM (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non‐stressed mice. Prog Neuropsychopharmacol Biol Psychiatry 64: 27–34. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR (1996). Effects of chronic treatment with Δ9‐tetrahydrocannabinol on cannabinoid‐stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci 16: 8057–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Roffman A (2006). The nature, consequences and treatment of cannabis dependence: implications for future research and policy In: Roffman A, Stephens R. (eds). Cannabis Dependence: Its Nature, Consequences and Treatment. Cambridge University Press: Cambridge: 343–356. ISBN:9780511544248. [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro . Br J Pharmacol 150: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B et al (2016a). Effects of fixed or self‐titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend 161: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Soliman A, Staios G, Quilty L, Fischer B, George TP et al (2016b). Sativex associated with behavioral‐relapse prevention strategy as treatment for cannabis dependence: a case series. J Addict Med 10: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2016). World drug report. edn: Vienna.
- Viudez‐Martinez A, Garcia‐Gutierrez MS, Navarron CM, Morales‐Calero MI, Navarrete F, Torres‐Suarez AI et al (2018). Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biol 23: 154–164. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR (2014). Adverse health effects of marijuana use. N Engl J Med 370: 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR (1995). Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology 34: 669–676. [DOI] [PubMed] [Google Scholar]
- Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR (2010). Antidepressant‐like effects of cannabidiol in mice: possible involvement of 5‐HT1A receptors. Br J Pharmacol 159: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R (1995). Antipsychotic effect of cannabidiol. J Clin Psychiatry 56: 485–486. [PubMed] [Google Scholar]


