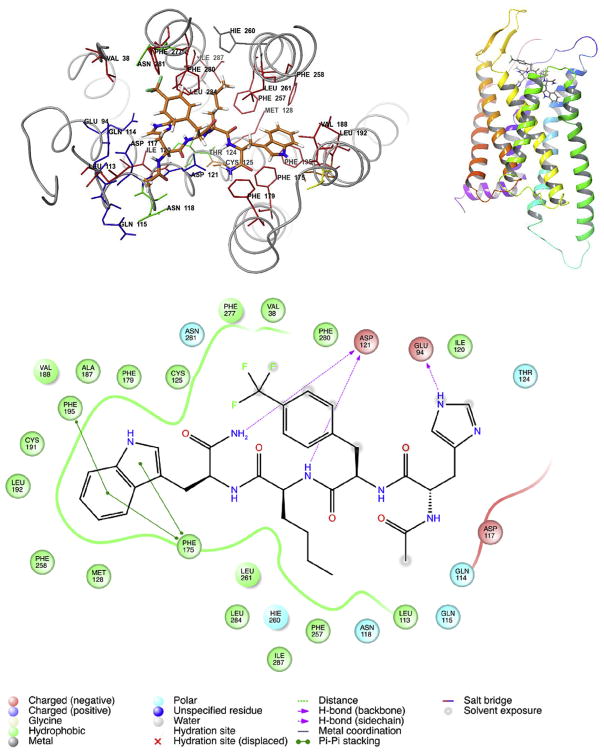
Fig. 4.
Best docking pose for hMC1R selective ligand: Ac-His-D-Phe(4-CF3)-Nle-Trp-NH2 into hMC1R. (Docking Score −11). The hMC1R-MCL interactions distance cut off 3 Å. Up Left: the selective molecule is highlight as tubing structure. The D-Phe(4-CF3) and Trp of the tetra peptide form π-π stacking interactions with the hMC1R transmembrane domains. Up Right: 3D view of selective molecule docking into hMC1R. Down: 2D structure of hMC1R-MCL interactions.