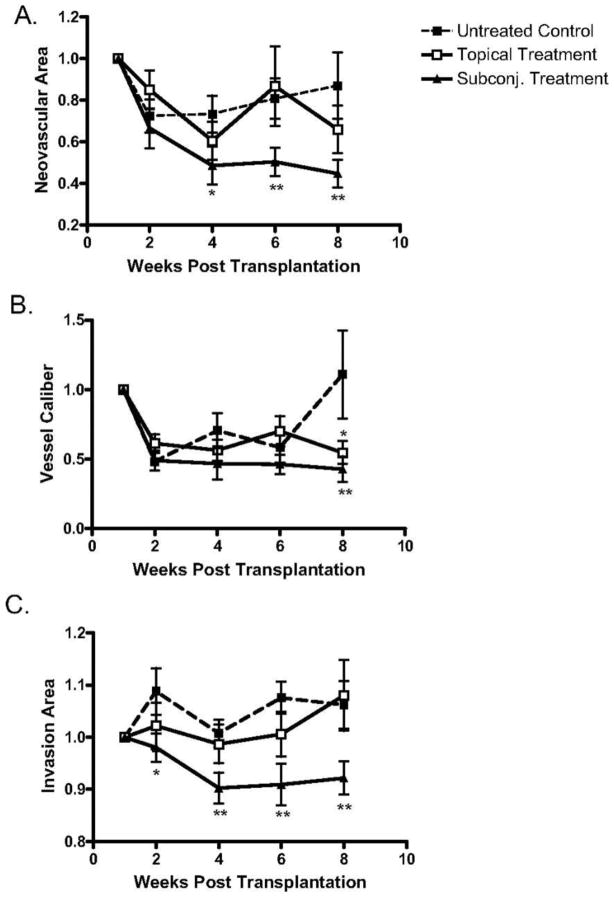
Figure 17.
Analysis of topical versus subconjunctival bevacizumab on the corneal NA, VC, and IA. High-risk graft beds in BALB/c mice were transplanted with C57BL/6 cornea, and mice were left untreated (n = 10) or were treated topically (n = 10) or subconjunctivally (n = 10) with bevacizumab. (A) Total area of blood vessels in each cornea was calculated and normalized to the baseline to yield the mean NA at the indicated time points to 8 weeks after transplantation. Although topical bevacizumab treatment mildly reduced NA in high-risk corneal transplantation, subconjunctival treatment resulted in a significant and marked reduction in NA at weeks 4, 6, and 8. (B) Normalized mean values for estimated blood vessel caliber at the indicated times to 8 weeks after transplantation. Although the subconjunctival treatment significantly reduced VC at week 8 (P = 0.03), topical bevacizumab appeared to have a marginal statistical difference from the control group (P = 0.05). (C) The total area of each given cornea invaded by blood vessels was calculated and normalized to yield the mean IA at the indicated times to 8 weeks after transplantation. Subconjunctival bevacizumab treatment appeared to be the only effective method to reduce IA. Student's t-test was performed to evaluate statistical significance (*P ≤ 0.05; **P < 0.01).53 (Adapted from Dastjerdi et al with permission from IOVS)