Figure 1.
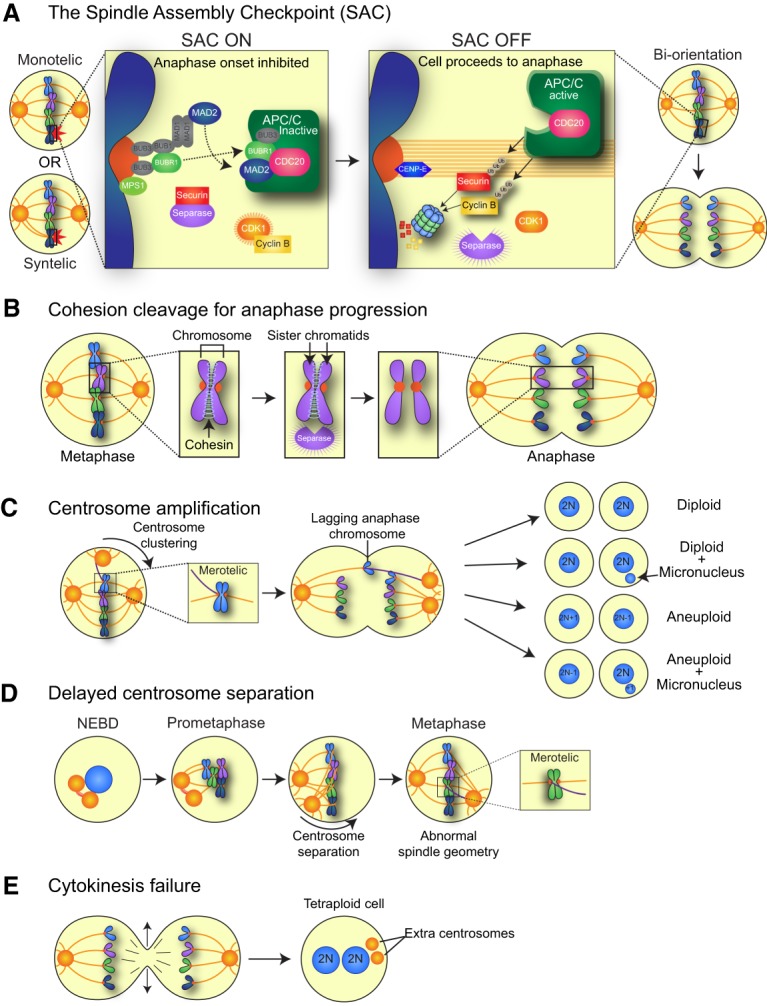
Chromosome segregation and sources of mitotic errors. (A) Unattached kinetochores activate an inhibitory SAC signal, which in turn blocks progression to anaphase. The target of the SAC is the APC/C, an E3 ubiquitin ligase that targets several proteins for degradation, including Cyclin B1 and Securin. When all kinetochores are correctly attached to MTs emerging from opposite poles of the cell (biorientation), the SAC is silenced, and APC/CCDC20 ubiquitinates and targets for degradation Cyclin B (to inactivate CDK1 and allow for mitotic exit) and Securin (to liberate the protease Separase and initiate the onset of anaphase). (B) Replicated sister chromatids are held together by the cohesin complex of proteins. Following silencing of the SAC, Securin is degraded, and the protease Separase is activated. Separase cleaves the cohesin complex to allow for sister chromatid separation and anaphase onset. (C) Extra centrosomes can generate a transient multipolar spindle, which, following centrosome clustering, leads to an increased rate of merotelic attachments, where one sister kinetochore is attached to MTs emerging from opposite poles. Merotelically attached chromosomes can lag in the middle of the spindle during anaphase and may subsequently be missegregated or incorporated into micronuclei. (D) After centrosome duplication, the two centrosomes are attached by a protein linker. This linker is disassembled prior to mitotic entry to allow the centrosomes to migrate apart and form opposite poles of the spindle. Delays in centrosome separation can lead to misattached chromosomes and/or abnormal spindle geometry that results in increased rates of chromosome missegregation. (E) Cleavage furrow regression leads to cytokinesis failure and the formation of a binucleate tetraploid cell with twice the normal centrosome content.
