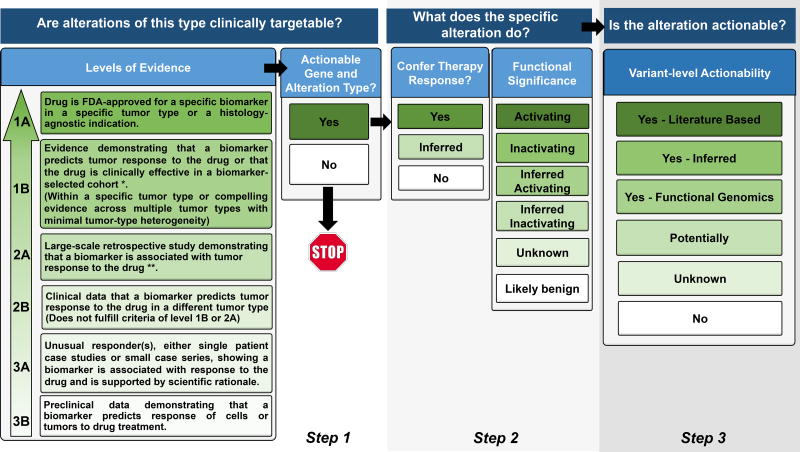
Figure 2.
Procedural flow used by the Precision Oncology Decision Support (PODS) team at MD Anderson Cancer Center for annotating an alteration. Our tiers for Levels of Evidence, Functional Significance, and Variant Level of Actionability are included. Terminology is defined in Supplemental Table 1.
* For Level of Evidence 1B, “Evidence” could be:
- An adequately-powered, prospective study with biomarker selection/stratification
- A meta-analysis/overview
- A consensus recommendation for standard of care (as recommended by NCCN guidelines or other consortia)
** For Level of Evidence 2A, “Evidence” could be:
- A prospective trial where biomarker study is the secondary objective
- An adequately-powered retrospective cohort study
- An adequately-powered case-control study