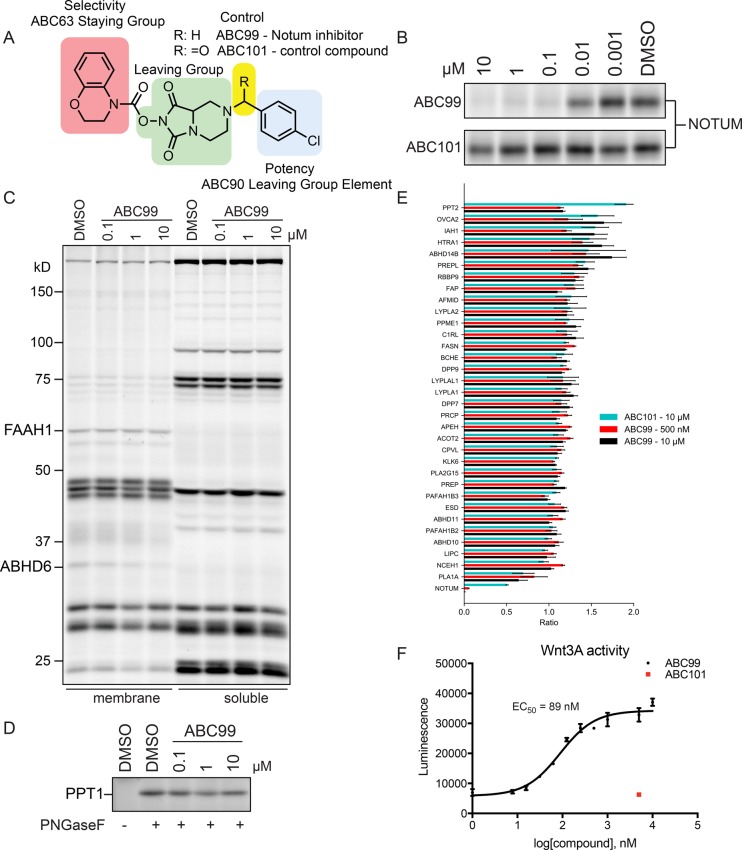
Figure 2.
Generation and characterization of ABC99, a potent and selective NOTUM inhibitor. (A) Structure of ABC99 and inactive control compound ABC101. (B) Concentration-dependent inhibition of NOTUM by ABC99 and ABC101 as measured by competitive ABPP using SW620 CM. (C,D) Competitive ABPP of SW620 cells treated in situ with ABC99 (indicated concentrations, 2 h, 37 °C) followed by exposure to the general serine hydrolase probe FP-Rh (C) or the PPT1-directed probe ABC45 (D). (E) Quantitative MS-based ABPP of SW620 CM treated with ABC99 (0.5 or 10 μM, 1 h, 37 °C), ABC101 (10 μM, 1 h, 37 °C), or DMSO. Serine hydrolases were enriched with FP-biotin (4 μM, 1 h, room temperature) and streptavidin chromatography. After on-bead trypsin digestion, peptides were isotopically labeled with heavy (DMSO) or light (compound) formaldehyde, then combined and processed for MS-based analysis.24 Ratios are displayed as light/heavy; therefore, low values indicate inhibition. Data for each experimental group represent median aggregate peptide ratios across two independent experiments +/− SEM. (F) ABC99, but not ABC101, preserves Wnt-3A activity in the presence of NOTUM in a concentration-dependent manner as measured using a Super TOPflash assay. Media from Wnt-3A expressing L-cells were incubated with inhibitor treated media from SW620 cells and then added to HEK293T-STF cells, which express luciferase in response to activation of the canonical Wnt pathway. Data represent average values with confidence intervals (CI) provided for four independent experiments.