FIGURE 4:
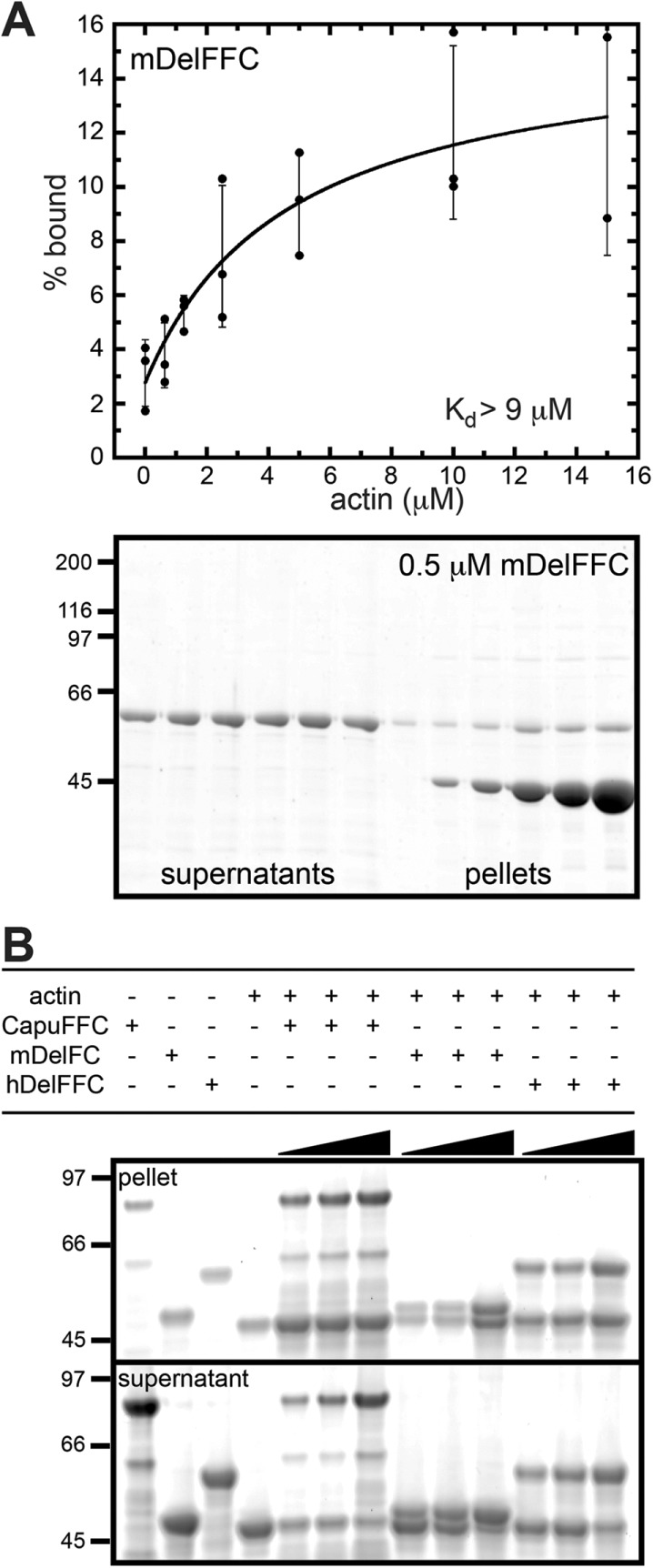
Delphilin is a weak actin bundler. (A) Using high-speed cosedimentation, we measured mDelFFC binding to actin filament sides. Various amounts of actin (0–15 μM) were mixed with 0.5 μM mDelFFC for 30 min at 25°C. Data from three independent experiments are shown. The line is a fit to averages of the data. The Kd reported (>9 μM) is the average of fits to the three independent experiments. Because the data do not reach a plateau, 9 μM is a lower limit of the Kd. At the bottom is a representative gel from a high-speed cosedimentation assay. (B) Low-speed sedimentation assays demonstrate that mDelFC and hDelFFC bundle actin filaments. Consistent with weak side binding, bundling is weak compared with CapuFFC. Various amounts of formin (1.25, 2.5, and 5 μM) were incubated with 5 μM F-actin for 30 min at 25°C. Bundles were sedimented by centrifugation at 12,000 × g for 15 min at 4°C.
