FIGURE 6:
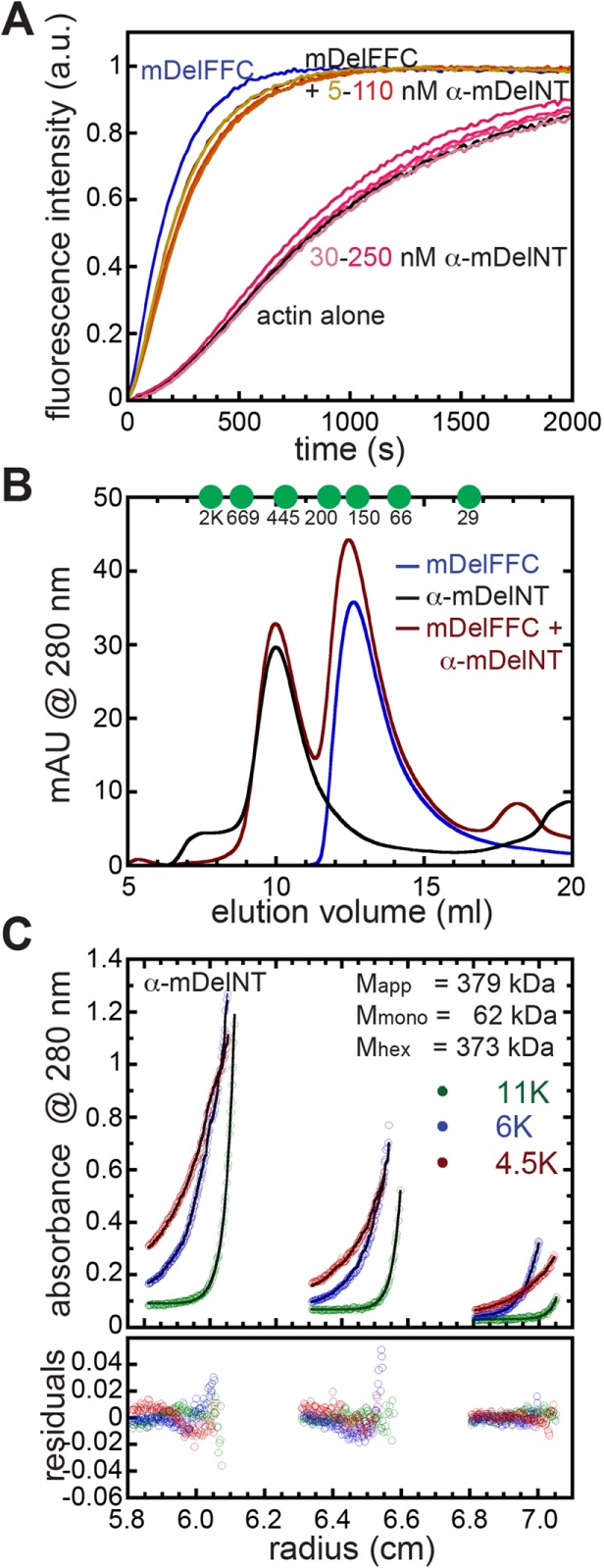
Delphilin is not autoinhibited. (A) The N-terminal half of α-Delphilin (α-mDelNT) has no effect on actin assembly whether or not mDelFFC is present. Conditions: 4 μM actin (10% pyrene labeled), 30 nM mDelFFC, and a range of α-mDelNT concentrations, as indicated. (B) Size exclusion chromatography was performed on mDelFC (blue), α-mDelNT (black), or both (red) with a Superdex S200 30/100 GL column. No differences were detected when the two proteins were mixed vs. alone. Elution peaks of standards are indicated with green circles. y-Axis is milli-absorbance units (mAU) measured at 280 nm. (C) Equilibrium sedimentation data for three concentrations of α-mDelNT at three speeds. Lines and residuals represent a global fit with a single species model. The data indicate that α-mDelNT is a hexamer. Values for the apparent mass (Mapp) and the masses predicted for α-mDelNT monomers (Mmono) and hexamers (Mhex) are given.
