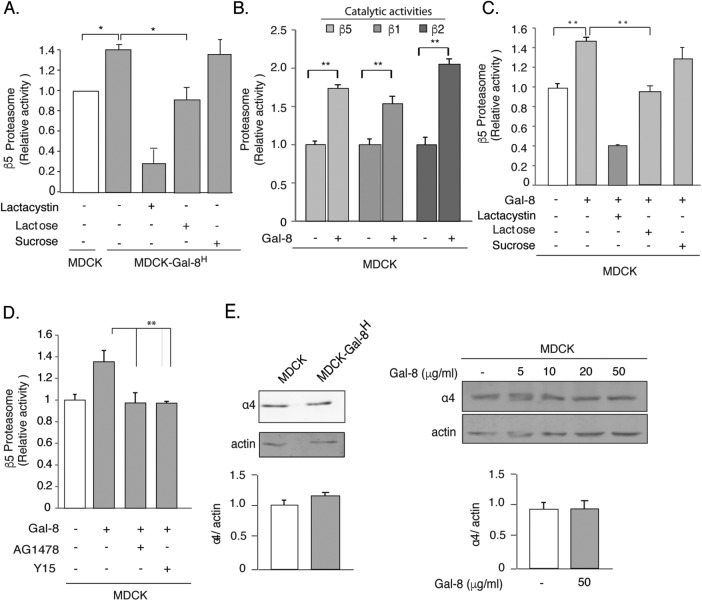
FIGURE 7:
Gal-8 increases the activity but not the expression of the proteasome through the FAK/EGFR pathway. (A) MDCK-Gal-8H cells show higher proteasome activity than MDCK cells, which is lowered by 20 mM β-lactose (but not by 20 mM sucrose) and by the proteasome inhibitor Lactacystin (1 μM). (B) Gal-8 (50 μg/ml) treatment for 4 h increases the chymotrypsin-, trypsin-, and caspase-like activity, corresponding to the proteasomal β5, β2, and β1 catalytic subunits. (C) Lactose (20 mM) (but not sucrose), and lactacystin counteract the Gal-8–induced increase of the proteasomal β5 catalytic activity. (D) Inhibition of EGFR (AG1478) and FAK (Y15) decrease the proteasome activity of MDCK-Gal-8H cells to the levels of MDCK cells (mean ± SEM; n = 9 wells from three experiments; *p < 0.05, **p < 0.005, one-way ANOVA). (E) Gal-8 does not change the proteasome expression. The α4 subunit of the 20S proteasome shows similar levels in MDCK-Gal-8H and MDCK, as well as in MDCK cells treated with 5, 10, 20, and 50 μg/ml Gal-8. Graphs show the mean ± SEM densitometric intensity from three experiments.