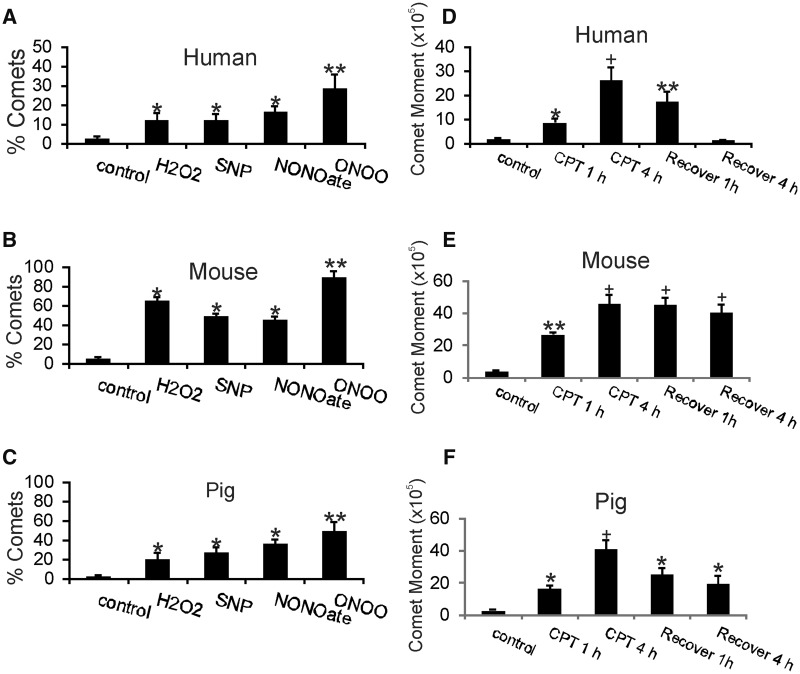
FIGURE 5.
Human, mouse, and pig neurons respond differently to oxidative and nitrative stress and have different DNA repair kinetics. (A–C) Cultured differentiated neurons were challenged with hydrogen peroxide (H2O2), nitric oxide donors (sodium nitroprusside [SNP] and NONOate) and peroxynitrite (ONOO) for 30 mins and then subjected to comet assay. Values are mean ± SD. Human neurons (A), mouse neurons (B), and pig neurons (C) all developed DNA damage after exposures to ROS and reactive nitrogen species. All types of neurons were most sensitive to ONOO because more DNA damage was found these groups. DNA damage accumulations in human and pig neurons were similar with exposure to H2O2, SNP, and NONOate (*p < 0.05), while mouse neurons had more severe DNA damage accumulation (*p < 0.01) compared to human and pig neurons. (D–F) Human neurons have more efficient DNA single-strand break (SSB) repair compared to mouse and pig neurons in cell culture. Differentiated human neurons (D), mouse neurons (E), and pig neurons (F) neurons were challenged with 10 μm CPT for 1 or 4 hours, washed, allowed to recover for 1 or 4 hours, and then subjected to comet assay to determine levels of DNA single-strand break repair as calculated by the comet moment (DNA intensitytail×tail area). Values are mean ± SD. Differentiated human neurons (D) treated with CPT accumulated significant levels of DNA damage (*p < 0.05; +p < 0.001 compared to vehicle-treated control) and showed efficient time-related repair DNA-SSBs (**p < 0.01). In contrast, CPT-treated mouse neurons (E) accumulated greater amounts of DNA damage compared to CPT-treated human neurons (**p < 0.01; +p < 0.001 compared to control) and were not successful at repairing DNA damage over a 4-hour recovery period. CPT-treated pig neurons (F) also accumulated significant levels of DNA damage (*p < 0.05; +p < 0.001 compared to vehicle-treated control neurons) and were significantly successful at repairing DNA (*p < 0.05).