Abstract
Coral-dinoflagellate symbiosis is the key biological interaction enabling existence of modern-type coral reefs, but the mechanisms regulating initial host–symbiont attraction, recognition and symbiont proliferation thus far remain largely unclear. A common reef-building coral, Acropora millepora, displays conspicuous fluorescent polymorphism during all phases of its life cycle, due to the differential expression of fluorescent proteins (FPs) of the green fluorescent protein family. In this study, we examine whether fluorescent variation in young coral juveniles exposed to natural sediments is associated with the uptake of disparate Symbiodinium assemblages determined using ITS-2 deep sequencing. We found that Symbiodinium assemblages varied significantly when redness values varied, specifically in regards to abundances of clades A and C. Whether fluorescence was quantified as a categorical or continuous trait, clade A was found at higher abundances in redder juveniles. These preliminary results suggest juvenile fluorescence may be associated with Symbiodinium uptake, potentially acting as either an attractant to ecologically specific types or as a mechanism to modulate the internal light environment to control Symbiodinium physiology within the host.
Keywords: Acropora millepora, Fluorescence, Symbiodinium, ITS-2
Introduction
The establishment of an obligate symbiosis between scleractinian corals and dinoflagellates of the genus Symbiodinium is ultimately critical for host survival and tolerance to environmental stressors (Fitt et al., 2001; Hennige et al., 2011). In species with environmental acquisition of Symbiodinium (horizontal transmission), larvae and recently metamorphosed coral hosts are exposed to a diversity of Symbiodinium. However critical, few studies have examined mechanisms of Symbiodinium uptake and acquisition by the host under natural conditions (Davy, Allemand & Weis, 2012). Establishment of symbiosis involves initial attraction, uptake of a variable but specific assemblage of Symbiodinium followed by winnowing (Little, Van Oppen & Willis, 2004; Rodriguez-Lanetty, Phillips & Weis, 2006; Abrego, Van Oppen & Willis, 2009a; Yamashita et al., 2014; Quigley, Bay & Willis, 2017). The mechanisms responsible for dinoflagellate-aposymbiotic host contact have been described as broadly chemotactic. However, these studies have been done in a few cnidarian species, including the upside-down jellyfish Cassiopia xamachana (Fitt & Barbara, 1984), mushroom coral Fungia scutaria (Hagedorn et al., 2015), and soft coral Heteroxenia fuscescens (Pasternak et al., 2004) but never in broadcast spawning scleractinians with positively buoyant larvae, extended competency periods and survivorship, as in Acropora millepora (Baird, 2001; Graham et al., 2013). In larvae of F. scutaria, trehalose excreted from Symbiodinium attracts and initiates feeding behaviour, a potential first step in initiating symbiosis (Hagedorn et al., 2015). Adult corals also seed the surrounding sediments with Symbiodinium, with an up to 8-fold increase in symbiont abundance in the sediments due to adult seeding (Nitschke, Davy & Ward, 2015). Young, negatively buoyant F. scutaria larvae are subsequently attracted to adult-seeded sediments (Hagedorn et al., 2015). In this scenario, larvae likely recruit and uptake symbionts very close to their parental colonies. However, in species predicted to disperse greater distances, such as Acropora millepora, symbiont uptake likely occurs after metamorphosis to ensure association with locally adapted symbiont types (Howells et al., 2012), potentially through non-chemosensory mechanisms (Hagedorn et al., 2015).
Fluorescence, one of the most conspicuous coral traits, is caused by the expression of fluorescent proteins (FPs). Expression of FPs is strongly regulated in response to environmental perturbations (D’Angelo et al., 2008; Bay et al., 2009; Aranda et al., 2011; DeSalvo et al., 2012; Roth, Fan & Deheyn, 2013), yet the biological functions of different spectral types of FPs in corals remain unclear. Acropora millepora exhibits conspicuous fluorescence polymorphism in larval, juvenile, and adult stages (Beltran-Ramirez, 2010; Kenkel et al., 2011; Strader, Aglyamova & Matz, 2018). A variety of hypotheses regarding the role of FPs on coral performance exist including, but not limited to, FPs acting as photoprotective molecules, dissipating excess light energy in shallow water habitats (Salih et al., 2000; Gittens et al., 2015), although this hypothesis is not universally supported (Mazel et al., 2003; Roth et al., 2015). Coral fluorescence interacts with symbiosis in a number of ways. In particular, specific FPs are known to be up-regulated during the initiation of symbiosis (Voolstra et al., 2009). The type of symbiont acquired by coral juveniles affects the abundance of green FP: juveniles exposed to non-infective C1 type Symbiodinium are significantly more green than juveniles exposed to type D Symbiodinium or aposymbiotic juveniles without symbionts (Yuyama & Higuchi, 2014). Expression of FPs under thermal stress also varies amongst juveniles hosting different symbiont types. Red and green FP expression increases during thermal stress in juveniles infected with type D symbionts and decreases in those infected with type C1 symbionts (Yuyama, Watanabe & Takei, 2011).
Fluorescent proteins can also modulate the internal host light environment by transforming the light spectrum and scattering light (Salih et al., 2000). Symbiodinium photosynthesis operates most efficiently under blue light in tested Symbiodinium types (Kinzie, Jokiel & York, 1984) yet Symbiodinium exhibit different physiologies under varying light regimes (Iglesias-Prieto & Trench, 1994), depths (Rowan et al., 1997; Bongaerts et al., 2010) and within coral individuals (Kemp, Fitt & Schmidt, 2008). Therefore, it is possible there are varying preferences among Symbiodinium types for differences in light intensity available for photosynthesis, which could be modulated by host fluorescence.
Furthermore, while some species of dinoflagellates move in response to light (Cullen, 1985; Horiguchi et al., 1999), it is unclear whether Symbiodinium are able to detect and move toward particular wavelengths emitted by coral juveniles. For example, using differential juvenile fluorescent intensities, signals and patterns for inter/intra-specific communication similar to what has been suggested for corals and reef fish (Matz, Marshall & Vorobyev, 2006; Lagorio, Cordon & Iriel, 2015). The majority of studies examining fluorescence in corals have targeted the adult stage, with considerably fewer studies examining the role of fluorescence in coral juveniles prior to the onset of symbiosis (Leutenegger et al., 2007; Roth et al., 2007; Kenkel et al., 2011; Roth, Fan & Deheyn, 2013; Strader, Aglyamova & Matz, 2016). It is currently unclear if corals use variable fluorescence signals that could, for example, allow for the attraction of commensal bacteria or their dinoflagellate symbionts, Symbiodinium.
To examine the relationship between coral juvenile fluorescence and the establishment of their symbiotic community, we employed next-generation sequencing of the ITS-2 region to genotype the Symbiodinium diversity present in Acropora millepora juveniles of varying fluorescence emissions after one month of exposure to sediments. This paper presents evidence for mostly overlapping yet subtly distinct Symbiodinium assemblages between green and red juvenile color morphs and discusses potential ecological drivers behind these differences.
Methods
Spawning and larval culturing
Eggs and sperm were acquired from eight gravid A. millepora colonies collected from Trunk Reef from about 3 metres depth (AIMS permit number: G12/35236.1) in November 2014 following spawning methods previously described in (Quigley, Willis & Bay, 2016). Briefly, positively-buoyant bundles were collected from the colonies, gently mixed with gametes from all 8 colonies, and then left to sit for 1.5 h to allow for fertilization to occur. After this time, fertilized embryos were washed three times by repeated transfer to new fresh-seawater containing bins. Bulk mixtures of gametes were maintained and larvae were raised in tanks with constant aeration and flow through set at 27 °C. Fully competent larvae were exposed to ground, autoclaved crustose coralline algae, a natural known settlement cue (Heyward & Negri, 1999), in sterile, plastic 6-well plates and allowed to naturally metamorphose for ∼24 h.
Fluorescence microscropy/ image analysis/ fluorescence quantification
After metamorphosis, fluorescent images of each individual juvenile were taken using a fluorescent stereo-microscope MZ FL-III (Leica, Bannockburn, IL, USA) equipped with a Canon G6 camera. Photos and image analysis was approached as in (Kenkel et al., 2011; Strader, Davies & Matz, 2015; Strader, Aglyamova & Matz, 2016). Juvenile fluorescence was imaged using the double-bandpass F/R filter (Chroma no. 51004v2), a filter that detects red fluorescence produced from the coral host while excluding any chlorophyll fluorescence. In addition, photographs were imaged prior to treatment exposure to Symbiodinium, therefore red fluorescence in coral hosts is not confounded by potential chlorophyll fluorescence. Image analysis was performed using ImageJ (Schneider, Rasband & Eliceiri, 2012). For each individual photograph, raw integrated RGB values were calculated across the area of the juvenile. A reference RGB value was calculated within a fixed circular area adjacent to the juvenile in each photograph and was subtracted from measured RGB values for each individual. Individual redness values were calculated as the normalized red value divided by the normalized green value plus the normalized red value.
Symbiodinium acquisition and community sequencing
Post-imaging, the six-well plates with settled juveniles were added to sediment treatments for natural uptake of Symbiodinium in the lab in the National Sea Simulator (Seasim) at the Australian Institute of Marine Sciences, resulting in 21 days of cumulative exposure to sediments. Six-well plates with juveniles were floated approximately 5 cm above the sediments using porcelain weights. Flow-through aquaria were fed with 27.2 °C, 0.4 µM filtered seawater that each contained 1L of sediments, with turn-over of the 45L tanks occurring once per hour. During this time, juveniles were exposed to natural light at 50 µmol photon illumination. Given the high level of water filtration and average Symbiodinium size of >4 µm (LaJeunesse et al., 2005), all Symbiodinium taken up by juveniles are assumed to be of sediment origins. Ten and twelve juveniles (n = 22) were sampled for green and red color morphs respectively from four tanks (tank 1: three red, three green; tank 2: three red, one green, tank 3: three red, three green, tank 4: three red, three green), and preserved in 100% ethanol and stored at −20 °C until sample processing. DNA extraction and sequence read analysis from individual juveniles followed (Quigley, Willis & Bay, 2016). Briefly, nucleic acid isolation included an initial chemical lysis, mechanical lysis using 1 mm silica beads (MPBio, Santa Ana, CA, USA), precipitation and clean-up (Wilson et al., 2002). DNA was sent to the University of Texas at Austin’s Genomic Sequencing and Analysis Facility (USA) for paired-end Miseq sequencing (Illumina, San Diego, CA, USA) of the ITS-2 region (Pochon et al., 2001). Raw reads were cleaned and analysed using the USEARCH and UPARSE pipeline (v.7) and identified to the clade/type level using a custom Symbiodinium database constructed from the NCBI database (see Supplementary Information) (Altschul et al., 1990; Camacho et al., 2009; Edgar, 2013).
Statistical analysis
Cleaned reads were variance normalized to account for differing sequence depth between samples using ‘DESeq2’ in R (R Core Team, 2013; Love, Huber & Anders, 2014). The functions ‘metaMDS’, ‘ordiplot’, ‘ordihull’, and ‘orditorp’ from the ‘vegan’ package and ‘ggplot2’ were used to construct NMDS plots using a Bray-Curtis distance matrix on variance-normalized OTU abundance data (Wickham, 2009; Oksanen et al., 2013). Gradient plots of the smooth response variable “redness” values over NMDS space were constructed using the ‘ordisurf’ function in ‘vegan.” Discriminant analysis of principle components (DAPC) was used to identify which OTUs may have attributed to juvenile groupings by fluorescence using the package ‘adegenet’ (Jombart, 2008).
Permutational multivariate analysis of variance using Bray-Curtis distance and Permutation test for homogeneity of multivariate dispersions was used to determine if Symbiodinium assemblages differed significantly between red and green juveniles using the ‘adonis’ and ‘betadisper’ functions in ‘vegan.’ To assess if there were significant differences in stabilized abundances between different Symbiodinium clades and types between red and green juveniles, independent 2-group Mann–Whitney U Tests (MWU) were performed (at alpha = 0.05). MWU Tests were run using the ‘wilcox.test’ function in the base R ‘stats’ package, which are able to account for the lack of homogeneity of variance across samples. To assess if there were significant differences in stabilized abundances between different OTUs found within red and green juveniles, negative binomial generalized linear models in ‘DESeq2’, using significant Benjamini–Hochberg p-values, were performed. The ‘ordisurf’ function and the ‘vegan’ package were used to generate Generalized Additive Models to determine if Symbiodinium assemblages differed significantly across redness values (Wood, 2006; Wood, 2008). To test for significant associations between variance normalized abundances and redness values for each OTU, Spearman’s rho correlation coefficients and associated p-values were calculated per OTU using the ‘cor.test’ function in the base R ‘stats’ package. Sample sizes per test for clades and types given in Tables S1 and S2. MWU and Spearman rho correlation tests were also randomized and re-run to assess if significant p-values were false-positives.
Results
Fluorescent variation among juveniles
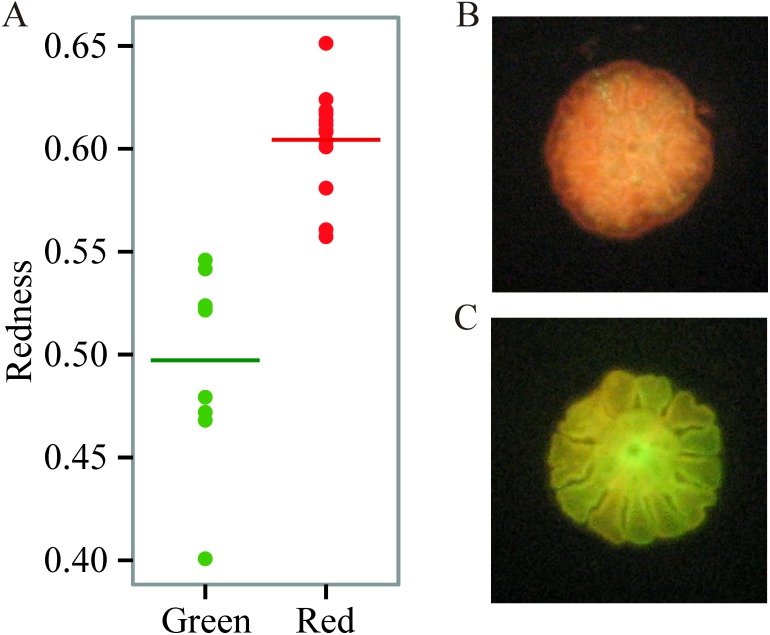
We found prominent variation in fluorescent phenotypes among young A. millepora juveniles (Fig. 1). Variation in fluorescence, quantified as ‘redness’, was continuous among individuals, however individuals were also binned into two categories and analysed as either ‘red’ or ‘green’ (Figs. 1; 2A, 2B). This fluorescent variation is due to variable expression of A. millepora GFP-like proteins, which vary in excitation/emission (Alieva et al., 2008; Beltran-Ramirez, 2010; Kenkel et al., 2011) and copy number (Gittens et al., 2015).
Figure 1. Variation in juvenile fluorescence.
(A) Fluorescence as a categorical trait (x-axis) and as a continuous trait (y- axis). (B) A red morph. (C) A green morph.
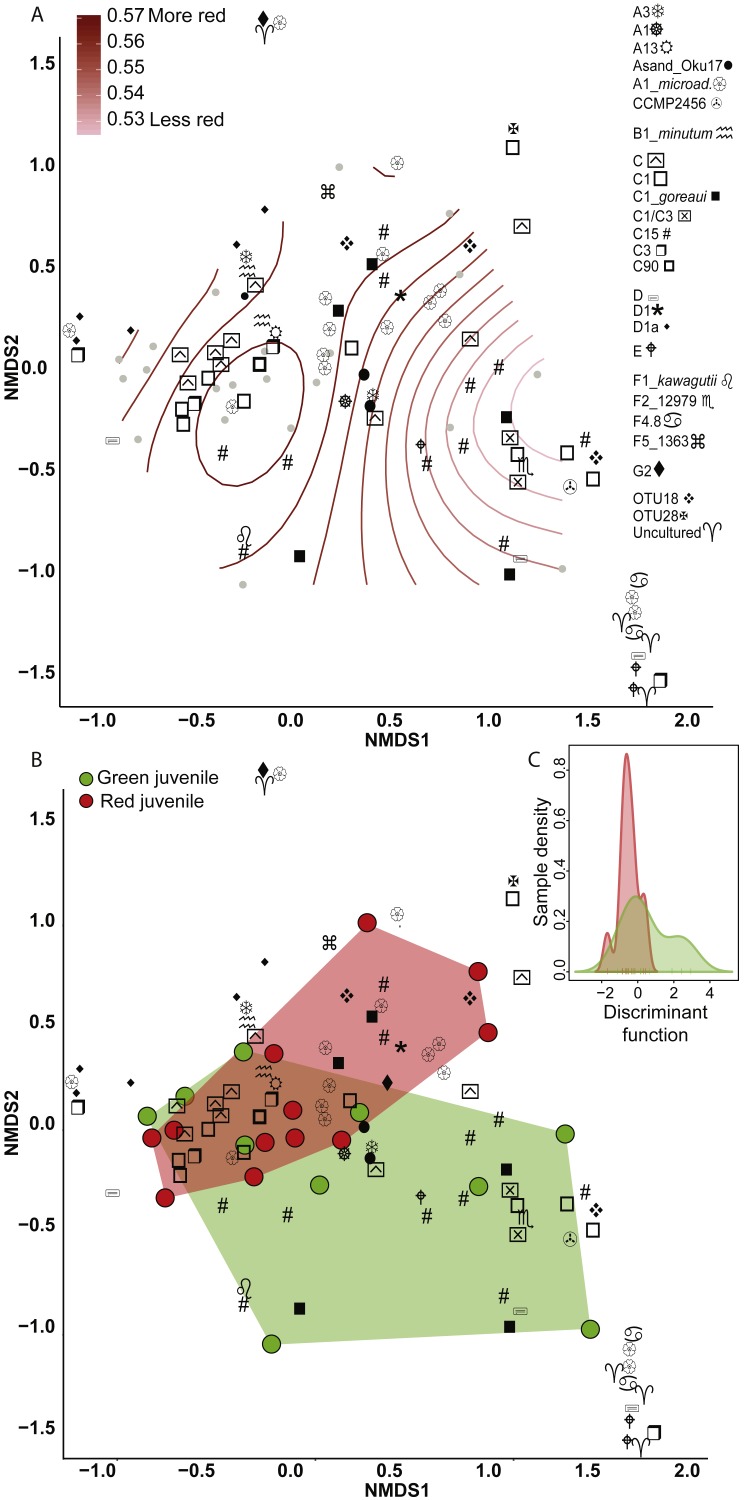
Figure 2. Non-metric multidimensional scaling (NMDS) using Bray Curtis distance matrix of variance normalized Symbiodinium abundances.
(A) Fluorescence as a categorical variable (red or green), and (B) as a continuous variable (redness). (C) Discriminant analysis of principle components (DAPC) with fluorescence as a categorical variable. Symbol shapes correspond to clade designations with multiple types (A, circular shapes; C, squares; D, star/varied shapes; F, line based shapes; Uncultured/OTUs, cross shapes).
ITS-2 variation between color morphs
Eighty-nine OTUs were recovered between the two color morphs (62 in red and 71 in green). When fluorescence was treated continuously, there was a marginally significant relationship between the Symbiodinium community and redness of A. millepora juveniles (Generalized Additive Model: p = 0.053, R2 = 0.33) (Fig. 2A). Of the 89 OTUs retrieved across juveniles, Spearman’s Rank Correlation tests indicated significant candidate OTUs as correlated with redness as a continuous variable (OTU79-C15, OTU985-A1; Fig. S1A). However, randomization tests (where redness values were shuffled among samples) indicated that these relationships might be false positives.
There was no significant relationship between Symbiodinium assemblage composition and fluorescence when color was treated as a categorical trait as found using either permutational manova (Adonis with Bray-Curtis distance: p = 0.58) (Fig. 2B) or discriminant analysis (Fig. 2C). OTU427 (C15) contributed the most to differentiating between the assemblages in the two color morphs as shown through loading weights of each OTU above discriminant analysis default thresholds (Figs. 2B, 2C). Although it appeared that green juveniles displayed a greater capacity to associate with a wider range of Symbiodinium OTUs and red juveniles displayed a more restricted assemblage (Figs. 2B, 2C), there was no significant relationship between the variances of the two color morphs (Permutation test for homogeneity of multivariate dispersions, df = 1, p = 0.5).
Clade and type abundances varied between color morphs (Fig. S1B), although after randomization, only clade A was robustly found in significantly greater abundances in red juveniles (Mann–Whitney U Tests, p = 0.03). The same OTU (427-C15) predicted as the OTU driving the greatest divergence of the two communities in the discriminant analysis was found at 15 fold lower abundances in red compared to green juveniles and OTU985 (S. microadriaticum) occurred in 74 fold higher abundance in red compared to green juveniles (DESeq2-Bejamini-Hochberg p-adjusted values both = 0.03).
Discussion
This study provides the first evidence of the potential role of fluorescence on the establishment of symbiosis in a broadcast spawning, horizontally transmitting reef-building coral. We find that Symbiodinium assemblages mostly overlap among juvenile fluorescent morphs with marginally significant separation between them when fluorescence is analysed as a continuous trait, driven mostly by variation in A1 and C15. These results emphasize that any future studies need to account for the continuous nature of fluorescent variation among recruits, rather than binning individuals into separate color categories. Despite our findings, there are substantial caveats in this study, notably the heritability of fluorescence in larvae of this species (Kenkel et al., 2011) and the fact that our experimental design did not disentangle potential parental effects. While our study is correlative, it opens the door for future studies to identify the causes of the relationship between juvenile fluorescent color and the initial Symbiodinium community.
Variable Symbiodinium types between color morphs
We detected significant changes in the Symbiodinium community as redness values shifted, potentially driven by significantly higher abundance of clade A in redder individuals and clade C in greener juveniles. Clade A is a generalist clade able to tolerate low and high light levels (Iglesias-Prieto & Trench, 1997a) and exhibits decreased growth in red compared to green light (Kinzie, Jokiel & York, 1984). C15 Symbiodinium often inhabit extreme shallow environments of high irradiance in species such as Montipora digitata, but can also dominate across substantial geography and depth in Porites spp. (Veron, 2000; LaJeunesse et al., 2003; LaJeunesse et al., 2004). It is possible that different abundances of specific Symbiodinium types in red and green fluorescent juveniles and significant changes to assembly composition with redness was observed because variation in the host internal light environment, or the availability of photosynthetically usable light, affects the proliferation or physiology of various Symbiodinium types differently. Spectral properties in coral tissues are regulated by FPs, with different FP types significantly changing the available wavelengths of light inside coral tissues (Salih et al., 2000). Green FP absorbs blue light and therefore diminishes the light available for photosynthesis and can suppress the cell-cycle of Symbiodinium (Kinzie, Jokiel & York, 1984; Alieva et al., 2008; Wang et al., 2008; Suggett et al., 2015). Therefore, GFP might act to regulate the amount of photosynthetically available light or proliferation of Symbiodinium, thus allowing uptake of an assemblage with specific physiologies. Alternatively, more red individuals are unable to regulate photosynthetically available light for Symbiodinium, which may promote the higher abundance of generalist clade A Symbiodinium and might reflect uptake of a more neutral Symbiodinium assemblage.
In addition, it is possible that greener host individuals are better at attracting more evolutionary derived C15 Symbiodinium as a way to avoid more opportunistic clade A Symbiodinium. Furthermore, opportunistic clade A Symbiodinium could avoid greener individuals as a means of increasing a competitive advantage with other Symbiodinium types. However, the actual dynamics of host/symbiont control regarding uptake and winnowing needs to be determined with follow-up studies. Regardless of what mechanisms may be at play, our results suggest subtle differences in Symbiodinium assemblages along a range of recruit fluorescence and drivers of these differences have yet to be investigated. It is also possible that the differences we observe may reflect variable irradiance preferences between Symbiodinium for specific light environments, although this is highly speculative and necessitates further study.
Differences in irradiance preferences may vary across specific Symbiodinium strains (Rowan et al., 1997), which could reflect differences in photo-acclimation potential. It is unclear how difference in irradiance preferences could impact coral performance, although perhaps this could occur through variability in carbon/nitrogen acquisition across strains, which occurs along depth gradients in Stylophora pistillata (Ezzat et al., 2017). Intra-clade level differences are extensive across a range of Symbiodinium traits and therefore the clade level may not be a good predictor of physiology. For example, in a comparative study, Symbiodinium microadriaticum and Symbiodinium pilosum (A2) had distinct photophysiological characteristics (Iglesias-Prieto & Trench, 1997b), and type A3 was found living in high irradiance environments through the production of MAA mycosporine-glycine (Banaszak et al., 2006). Photophysiological traits such as electron transport rates, cellular RCII concentrations, and light harvesting also differed across distinct types and did not cluster by clade; again suggesting that types within clades A and F are physiologically distinct (Iglesias-Prieto & Trench, 1997a; Suggett et al., 2015). Finally, the unique OTUs for both color morphs suggests that these are distinct subtypes and that variations between these types exist (for example: C3-u vs. C3-z prevalence between light environments (LaJeunesse et al., 2010).
It is possible that other physiological differences among juveniles besides fluorescence may be influencing Symbiodinium uptake. The higher abundances of specific Symbiodinium types may be due to different by-products produced by these two juvenile color morphs. The sugar trehalose has been found to be a strong chemical attractant for coral larvae (Hagedorn et al., 2015) and different Symbiodinium types may be similarly attracted to trehalose or other sugar derived chemicals. Potentially, different color morphs could produce variable attractive compounds or quantities. Indeed, the variable production of N-acetyl glucosamine and C6 sugars in coral mucus has been found to significantly alter bacterial assemblages (Lee et al., 2016). Finally, since our experiment was performed on a bulk culture containing multiple genotypes, it is also possible that the variation we see in regards to Symbiodinium could be a result of genotypic variation influencing uptake as there are heritable components to both fluorescence and Symbiodinium acquisition (Kenkel et al., 2011; Quigley, Willis & Bay, 2017). Further work is needed to determine if other traits aside from variable fluorescence are associated with juvenile physiology that may explain variable uptake of symbionts.
Although we found a significant correlation between the abundance of two specific OTUs representing two Symbiodinium types and redness fluorescence, a previous report found no significant relationship between symbiont abundance and green fluorescence in Seriatopora hystrix (Roth, Fan & Deheyn, 2013). This may be due to three reasons: (1) that study did not differentiate between different Symbiodinium clades or types, (2) all Seriatopora hystrix larvae measured were only of green fluorescence and did not exhibit red to green variability as measured in A. millepora, and (3) differing methods in Symbiodinium quantification. In addition, it is possible that fluorescence shifts during juvenile ontogeny, making comparisons between larval and juvenile fluorescence incongruent.
Outlook for future research
Despite the caveats in our results, this study highlights the potential of juvenile fluorescence to modulate the host light environment impacting the physiology of specific Symbiodinium types or function as an attractant for specific types of ecologically diverse Symbiodinium. Any studies going forward would need to incorporate larger sample sizes, as it is possible that the low number of sequenced animals in this study limited our power to distinguish between significant changes and noise in Symbiodinium types and OTUs variable between fluorescent morphs. The first step forward would be to disentangle potential parental effects of fluorescence and Symbiodinium assemblage uptake (Kenkel et al., 2011; Quigley, Willis & Bay, 2017). Despite potentially confounding parental effects, our study provides the first evidence that juvenile fluorescence is a trait potentially linked to mechanisms of Symbiodinium acquisition and warrants further investigation. In addition, it would be ideal to monitor the time course of symbiont winnowing among fluorescent color morphs, as the study presented here is merely a snapshot in time during a dynamic period of restructuring of Symbiodinium assemblages (Quigley, Bay & Willis, 2017). It is possible that the differences in Symbiodinium OTUs and types we observe in this study are due to differences in the timing of winnowing between color morphs (Abrego, Van Oppen & Willis, 2009b). If true, this would support a role of juvenile fluorescence in the winnowing process. It would also be ideal to disentangle if Symbiodinium acquisition is dependent on the light transforming properties of FPs by performing manipulative light experiments using specific filters to modulate the host fluorescence available to Symbiodinium prior to infection, as in (Strader, Davies & Matz, 2015). This would test the hypothesis of host fluorescence acting as an attractant, a hypothesis that has also been proposed in (Hollingsworth et al., 2005). It is imperative to investigate the spectral and behavioural properties of specific symbiont types, specifically characterizing the wavelengths of light that can be detected as well as irradiance preferences between specific Symbiodinium types (Suggett et al., 2015; Innis et al., 2018). For example, our data suggest that clade A could be less attracted to high abundances of GFP in juveniles, although the physiological response of clade A Symbiodinium to different light spectra and intensity need further substantiation. To take it a step further, it would be interesting to identify if different irradiance preferences were related to differential nutrient acquisition or other aspects of coral performance (Ezzat et al., 2017). Finally, our findings provide a framework for further experimental studies exploring the ecological function of fluorescence during coral ontogeny and its potential influence on the establishment of symbiosis.
Conclusions
To the best of our knowledge, this is the first study to examine the potential function of coral fluorescent proteins in modulating the uptake of Symbiodinium in broadcast spawning corals with horizontal symbiont acquisition. We find that in A. millepora, juvenile fluorescence varies continuously between green and red and this fluorescent variation is associated with preferential uptake of clade A in redder individuals and type C15 Symbiodinium in greener individuals. The biological significance of these associations has yet to be determined; however; we hope this work will provide a platform for future studies investigating the functional significance of the association between coral fluorescence and initial uptake of Symbiodinium assemblages.
Supplemental Information
(A) Barplots of clades/type/OTUs. (B) Scatterplots comparing the redness of juveniles (x-axis) to the variance normalized abundance of different Symbiodinium types (y-axis, depicted as whole numbers). Asterisks below clade designations on barplots are significantly correlated with red juveniles.
Sample size per Symbiodinium clade/category per treatment group. * denote NCBI given names to OTU taxonomies, not author designations.
* denote NCBI given names to OTU taxonomies, not author designations.
Funding Statement
Funding was provided by the Australian Research Council through ARC CE1401000020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Kate M. Quigley, Email: katemarie.quigley@my.jcu.edu.au.
Marie E. Strader, Email: stradermarie@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kate M. Quigley conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Marie E. Strader conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Mikhail V. Matz contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments were approved by the Great Barrier Reef Marine Park Authority (GBRMPA) (Permit Number G12/35236.1).
Data Availability
The following information was supplied regarding data availability:
Sequences are available using the SRA accession: SRP133664.
References
- Abrego, Van Oppen & Willis (2009a).Abrego D, Van Oppen MJH, Willis BL. Highly infectious symbiont dominates initial uptake in coral juveniles. Molecular Ecology. 2009a;18:3518–3531. doi: 10.1111/j.1365-294X.2009.04275.x. [DOI] [PubMed] [Google Scholar]
- Abrego, Van Oppen & Willis (2009b).Abrego D, Van Oppen MJH, Willis BL. Onset of algal endosymbiont specificity varies among closely related species of Acropora corals during early ontogeny. Molecular Ecology. 2009b;18:3532–3543. doi: 10.1111/j.1365-294X.2009.04276.x. [DOI] [PubMed] [Google Scholar]
- Alieva et al. (2008).Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V, Miller DJ, Wiedenmann J, Salih A, Matz MV. Diversity and evolution of coral fluorescent proteins. PLOS ONE. 2008;3:e2680. doi: 10.1371/journal.pone.0002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aranda et al. (2011).Aranda M, Banaszak AT, Bayer T, Luyten JR, Medina M, Voolstra CR. Differential sensitivity of coral larvae to natural levels of ultraviolet radiation during the onset of larval competence. Molecular Ecology. 2011;20:2955–2972. doi: 10.1111/j.1365-294X.2011.05153.x. [DOI] [PubMed] [Google Scholar]
- Baird (2001).Baird AH. Doctoral dissertation. 2001. The ecology of coral larvae: settlement patterns, habitat selection and the length of the larval phase. [Google Scholar]
- Banaszak et al. (2006).Banaszak AT, Santos MG, LaJeunesse TC, Lesser MP. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. Journal of Experimental Marine Biology and Ecology. 2006;337:131–146. doi: 10.1016/j.jembe.2006.06.014. [DOI] [Google Scholar]
- Bay et al. (2009).Bay LK, Ulstrup KE, Nielsen HB, Jarmer H, Goffard N, Willis BL, Miller DJ, Van Oppen MJH. Microarray analysis reveals transcriptional plasticity in the reef building coral Acropora millepora. Molecular Ecology. 2009;18:3062–3075. doi: 10.1111/j.1365-294X.2009.04257.x. [DOI] [PubMed] [Google Scholar]
- Beltran-Ramirez (2010).Beltran-Ramirez V. PhD thesis. 2010. Molecular aspects of the fluorescent protein homologues in Acropora millepora. [Google Scholar]
- Bongaerts et al. (2010).Bongaerts P, Riginos C, Ridgway T, Sampayo EM, Van Oppen MJH, Englebert N, Vermeulen F, Hoegh-Guldberg O. Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLOS ONE. 2010;5:e10871. doi: 10.1371/journal.pone.0010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho et al. (2009).Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen (1985).Cullen JJ. Diel vertical migration by dinoflagellates: roles of carbohydrate metabolism and behavioral flexibility. Contributions in Marine Science. 1985;27:135–152. [Google Scholar]
- D’Angelo et al. (2008).D’Angelo C, Denzel A, Vogt A, Matz M, Oswald F, Salih A, Nienhaus G, Wiedenmann J. Blue light regulation of host pigment in reef-building corals. Marine Ecology Progress Series. 2008;364:97–106. doi: 10.3354/meps07588. [DOI] [Google Scholar]
- Davy, Allemand & Weis (2012).Davy SK, Allemand D, Weis VM. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews. 2012;76:229–261. doi: 10.1128/MMBR.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo et al. (2012).DeSalvo MK, Estrada A, Sunagawa S, Medina M. Transcriptomic responses to darkness stress point to common coral bleaching mechanisms. Coral Reefs. 2012;31:215–228. doi: 10.1007/s00338-011-0833-4. [DOI] [Google Scholar]
- Edgar (2013).Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Ezzat et al. (2017).Ezzat L, Fine M, Maguer J-F, Grover R, Ferrier-Pagès C. Carbon and nitrogen acquisition in shallow and deep holobionts of the scleractinian coral S. pistillata. Frontiers in Marine Science. 2017;4 doi: 10.3389/fmars.2017.00102. Article 102. [DOI] [Google Scholar]
- Fitt & Barbara (1984).Fitt WK, Barbara S. The role of chemosensory behavior of Symbiodinium microadriaticum, intermediate hosts, and host behavior in the infection of coelenterates and molluscs with zooxanthellae. Marine Biology. 1984;81:9–17. doi: 10.1007/BF00397620. [DOI] [Google Scholar]
- Fitt et al. (2001).Fitt WK, Brown BE, Warner ME, Dunne RP. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. doi: 10.1007/s003380100146. [DOI] [Google Scholar]
- Gittens et al. (2015).Gittens JR, D’Angelo C, Oswald F, Edwards RJ, Wiedenmann J. Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Molecular Ecology. 2015;24:453–465. doi: 10.1111/mec.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham et al. (2013).Graham EM, Baird AH, Willis BL, Connolly SR. Effects of delayed settlement on post-settlement growth and survival of scleractinian coral larvae. Oecologia. 2013;173:431–438. doi: 10.1007/s00442-013-2635-6. [DOI] [PubMed] [Google Scholar]
- Hagedorn et al. (2015).Hagedorn M, Carter V, Zuchowicz N, Phillips M, Penfield C, Shamenek B, Vallen EA, Kleinhans FW, Peterson K, White M, Yancey PH. Trehalose is a chemical attractant in the establishment of coral symbiosis. PLOS ONE. 2015;10:e0117087. doi: 10.1371/journal.pone.0117087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennige et al. (2011).Hennige SJ, McGinley MP, Grottoli AG, Warner ME. Photoinhibition of Symbiodinium spp. within the reef corals Montastraea faveolata and Porites astreoides: implications for coral bleaching. Marine Biology. 2011;158:2515–2526. doi: 10.1007/s00227-011-1752-1. [DOI] [Google Scholar]
- Heyward & Negri (1999).Heyward AJ, Negri AP. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. doi: 10.1007/s003380050193. [DOI] [Google Scholar]
- Hollingsworth et al. (2005).Hollingsworth LL, Kinzie RA, Lewis TD, Krupp DA, Leong J-AC. Phototaxis of motile zooxanthellae to green light may facilitate symbiont capture by coral larvae. Coral Reefs. 2005;24:523. doi: 10.1007/s00338-005-0063-8. [DOI] [Google Scholar]
- Horiguchi et al. (1999).Horiguchi T, Kawai H, Kubota M, Takahashi T, Watanabe M. Phototactic responses of four marine dinoflagellates with different types of eyespot and chloroplast. Phycological Research. 1999;47:101–107. doi: 10.1111/j.1440-1835.1999.tb00290.x. [DOI] [Google Scholar]
- Howells et al. (2012).Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, Van Oppen MJH. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nature Climate Change. 2012;2:116–120. [Google Scholar]
- Iglesias-Prieto & Trench (1994).Iglesias-Prieto R, Trench RK. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Marine Ecology-Progress Series. 1994;113:163–175. doi: 10.3354/meps113163. [DOI] [Google Scholar]
- Iglesias-Prieto & Trench (1997a).Iglesias-Prieto R, Trench RK. Photoadaptation, photoacclimation and niche diversification in invertebrate-dinoflagellate symbioses. Proc 8th int coral Reef Symp. 1997a;2:1319–1324. [Google Scholar]
- Iglesias-Prieto & Trench (1997b).Iglesias-Prieto R, Trench RK. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll–protein complexes to different photon-flux densities. Marine Biology. 1997b;130:23–33. doi: 10.1007/s002270050221. [DOI] [Google Scholar]
- Innis et al. (2018).Innis T, Cunning R, Ritson-Williams R, Wall CB, Gates RD. Coral color and depth drive symbiosis ecology of Montipora capitata in Kāne ‘ohe Bay, O ‘ahu, Hawai ‘i. Coral Reefs. 2018;37(2):423–430. [Google Scholar]
- Jombart (2008).Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- Kemp, Fitt & Schmidt (2008).Kemp DW, Fitt WK, Schmidt GW. A microsampling method for genotyping coral symbionts. Coral Reefs. 2008;27:289–293. doi: 10.1007/s00338-007-0333-8. [DOI] [Google Scholar]
- Kenkel et al. (2011).Kenkel CD, Traylor MR, Wiedenmann J, Salih A, Matz MV. Fluorescence of coral larvae predicts their settlement response to crustose coralline algae and reflects stress. Proceedings of the Royal Society B: Biological Sciences. 2011;278(1718):2691–2697. doi: 10.1098/rspb.2010.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzie, Jokiel & York (1984).Kinzie RA, Jokiel PL, York R. Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Marine Biology. 1984;78:239–248. doi: 10.1007/BF00393009. [DOI] [Google Scholar]
- Lagorio, Cordon & Iriel (2015).Lagorio MG, Cordon GB, Iriel A. Reviewing the relevance of fluorescence in biological systems. Photochemical & Photobiological Sciences. 2015;14:1538–1559. doi: 10.1039/C5PP00122F. [DOI] [PubMed] [Google Scholar]
- LaJeunesse et al. (2005).LaJeunesse TC, Lambert G, Andersen RA, Coffroth MA, Galbraith DW. Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among Dinoflagellates. Journal of Phycology. 2005;41:880–886. doi: 10.1111/j.0022-3646.2005.04231.x. [DOI] [Google Scholar]
- LaJeunesse et al. (2003).LaJeunesse TC, Loh WKW, Woesik R van, Hoegh-Guldberg O, Schmidt GW, Fitt WK. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnology and Oceanography. 2003;48:2046–2054. doi: 10.4319/lo.2003.48.5.2046. [DOI] [Google Scholar]
- LaJeunesse et al. (2010).LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK. Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. Journal of Biogeography. 2010;37:785–800. doi: 10.1111/j.1365-2699.2010.02273.x. [DOI] [Google Scholar]
- LaJeunesse et al. (2004).LaJeunesse TC, Thornhill DJ, Cox EF, Stanton FG, Fitt WK, Schmidt GW. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs. 2004;23:596–603. doi: 10.1007/s00338-004-0428-4. [DOI] [Google Scholar]
- Lee et al. (2016).Lee STM, Davy SK, Tang S-L, Kench PS. Mucus sugar content shapes the bacterial community structure in thermally stressed Acropora muricata. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00371. Article 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger et al. (2007).Leutenegger A, D’Angelo C, Matz MV, Denzel A, Oswald F, Salih A, Nienhaus GU, Wiedenmann J. It’s cheap to be colorful. FEBS Journal. 2007;274:2496–2505. doi: 10.1111/j.1742-4658.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Little, Van Oppen & Willis (2004).Little AF, Van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- Love, Huber & Anders (2014).Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:1–21. doi: 10.1186/gb-2014-15-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz, Marshall & Vorobyev (2006).Matz MV, Marshall NJ, Vorobyev M. Are corals colorful? Photochemistry and Photobiology. 2006;82:345–350. doi: 10.1562/2005-08-18-RA-653. [DOI] [PubMed] [Google Scholar]
- Mazel et al. (2003).Mazel CH, Strand MP, Lesser MP, Crosby MP, Coles B, Nevis AJ. High-resolution determination of coral reef bottom cover from multispectral fluorescence laser line scan imagery. Limnology and Oceanography. 2003;48:522–534. doi: 10.4319/lo.2003.48.1_part_2.0522. [DOI] [Google Scholar]
- Nitschke, Davy & Ward (2015).Nitschke MR, Davy SK, Ward S. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs. 2015;35(1):335–344. [Google Scholar]
- Oksanen et al. (2013).Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. Package ‘vegan’. version 2https://CRAN.R-project.org/package=vegan Community ecology package. 2013
- Pasternak et al. (2004).Pasternak Z, Bachar A, Abelson A, Achituv Y. Initiation of symbiosis between the soft coral Heteroxenia fuscescens and its zooxanthellae. Marine Ecology Progress Series. 2004;279:113–116. doi: 10.3354/meps279113. [DOI] [Google Scholar]
- Pochon et al. (2001).Pochon X, Pawlowski J, Zaninetti L, Rowan R. High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Marine Biology. 2001;139:1069–1078. doi: 10.1007/s002270100674. [DOI] [Google Scholar]
- Quigley, Bay & Willis (2017).Quigley KM, Bay LK, Willis BL. Temperature and water quality-related patterns in sediment-associated Symbiodinium communities impact symbiont uptake and fitness of juveniles in the genus Acropora. Frontiers in Marine Science. 2017;4 doi: 10.3389/fmars.2017.00401. Article 401. [DOI] [Google Scholar]
- Quigley, Willis & Bay (2016).Quigley KM, Willis BL, Bay LK. Maternal effects and Symbiodinium community composition drive differential patterns in juvenile survival in the coral Acropora tenuis. Royal Society Open Science. 2016;3:1–17. doi: 10.1098/rsos.160471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, Willis & Bay (2017).Quigley K, Willis B, Bay L. Heritability of the Symbiodinium community in vertically-and horizontally-transmitting broadcast spawning corals. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-08179-4. Article 8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team . R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- Rodriguez-Lanetty, Phillips & Weis (2006).Rodriguez-Lanetty M, Phillips WS, Weis VM. Transcriptome analysis of a cnidarian—dinoflagellate mutualism reveals complex modulation of host gene expression. BMC Genomics. 2006;7(1):23. doi: 10.1186/1471-2164-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth et al. (2007).Roth MS, Alamaru A, Padilla-Gamiño JL, Gates RD. Fluorescence in eggs of the coral Montipora capitata. In: Gates RD, editor. The biology of corals: developing a fundamental understanding of the coral stress response Final report of the 2007 Edwin W Pauley Summer Program in Marine Biology. Kaneohe, Hawaii: University of Hawai‘i; 2007. [Google Scholar]
- Roth, Fan & Deheyn (2013).Roth MS, Fan T-Y, Deheyn DD. Life history changes in coral fluorescence and the effects of light intensity on larval physiology and settlement in Seriatopora hystrix. PLOS ONE. 2013;8:e59476. doi: 10.1371/journal.pone.0059476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth et al. (2015).Roth MS, Padilla-Gamiño JL, Pochon X, Bidigare RR, Gates RD, Smith CM, Spalding HL. Fluorescent proteins in dominant mesophotic reef-building corals. Marine Ecology Progress Series. 2015;521:63–79. doi: 10.3354/meps11108. [DOI] [Google Scholar]
- Rowan et al. (1997).Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- Salih et al. (2000).Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000;408:850–853. doi: 10.1038/35048564. [DOI] [PubMed] [Google Scholar]
- Schneider, Rasband & Eliceiri (2012).Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader, Aglyamova & Matz (2016).Strader ME, Aglyamova GV, Matz MV. Red fluorescence in coral larvae is associated with a diapause like state. Molecular Ecology. 2016;25:559–569. doi: 10.1111/mec.13488. [DOI] [PubMed] [Google Scholar]
- Strader, Aglyamova & Matz (2018).Strader ME, Aglyamova GV, Matz MV. Molecular characterization of larval development from fertilization to metamorphosis in a reef-building coral. BMC genomics. 2018;19:17. doi: 10.1186/s12864-017-4392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader, Davies & Matz (2015).Strader ME, Davies SW, Matz MV. Differential responses of coral larvae to the colour of ambient light guide them to suitable settlement microhabitat. Royal Society Open Science. 2015;2 doi: 10.1098/rsos.150358. Article 150358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggett et al. (2015).Suggett DJ, Goyen S, Evenhuis C, Szabó M, Pettay DT, Warner ME, Ralph PJ. Functional diversity of photobiological traits within the genus Symbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytologist. 2015;208:370–381. doi: 10.1111/nph.13483. [DOI] [PubMed] [Google Scholar]
- Veron (2000).Veron JV. Corals of the World. Vol. 1–3. Townsville: Australian Institute of Marine Science; 2000. [Google Scholar]
- Voolstra et al. (2009).Voolstra CR, Schwarz JA, Schnetzer J, Sunagawa S, Desalvo MK, Szmant AM, Coffroth MA, Medina M. The host transcriptome remains unaltered during the establishment of coral-algal symbioses. Molecular Ecology. 2009;18:1823–1833. doi: 10.1111/j.1365-294X.2009.04167.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2008).Wang L, Liu Y, Ju Y, Hsiao Y, Fang L, Chen C. Cell cycle propagation is driven by light–dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral Reefs. 2008;27:823–835. doi: 10.1007/s00338-008-0434-z. [DOI] [Google Scholar]
- Wickham (2009).Wickham H. Berlin/Heidelberg: Springer Science & Business Media; 2009. [Google Scholar]
- Wilson et al. (2002).Wilson K, Li Y, Whan V, Lehnert S, Byrne K, Moore S, Pongsomboon S, Tassanakajon A, Rosenberg G, Ballment E. Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture. 2002;204:297–309. doi: 10.1016/S0044-8486(01)00842-0. [DOI] [Google Scholar]
- Wood (2006).Wood S. Generalized additive models: an introduction with R. Boca Raton: CRC press; 2006. [Google Scholar]
- Wood (2008).Wood S. Fast stable direct fitting and smoothness selection for generalized additive models. Journal of the Royal Statistical Society: Series B Statistical Methodology. 2008;70:495–518. doi: 10.1111/j.1467-9868.2007.00646.x. [DOI] [Google Scholar]
- Yamashita et al. (2014).Yamashita H, Suzuki G, Kai S, Hayashibara T, Koike K. Establishment of coral-algal symbiosis requires attraction and selection. PLOS ONE. 2014;9:e97003. doi: 10.1371/journal.pone.0097003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama & Higuchi (2014).Yuyama I, Higuchi T. Comparing the effects of symbiotic algae (Symbiodinium) clades C1 and D on early growth stages of Acropora tenuis. PLOS ONE. 2014;9:e98999. doi: 10.1371/journal.pone.0098999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama, Watanabe & Takei (2011).Yuyama I, Watanabe T, Takei Y. Profiling differential gene expression of symbiotic and aposymbiotic corals using a high coverage gene expression profiling (HiCEP) analysis. Marine biotechnology. 2011;13:32–40. doi: 10.1007/s10126-010-9265-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Barplots of clades/type/OTUs. (B) Scatterplots comparing the redness of juveniles (x-axis) to the variance normalized abundance of different Symbiodinium types (y-axis, depicted as whole numbers). Asterisks below clade designations on barplots are significantly correlated with red juveniles.
Sample size per Symbiodinium clade/category per treatment group. * denote NCBI given names to OTU taxonomies, not author designations.
* denote NCBI given names to OTU taxonomies, not author designations.
Data Availability Statement
The following information was supplied regarding data availability:
Sequences are available using the SRA accession: SRP133664.