Figure 5. Neutralizing antibody ADI-19425 uses germline-encoded features for high-affinity binding to antigenic site III on preF.
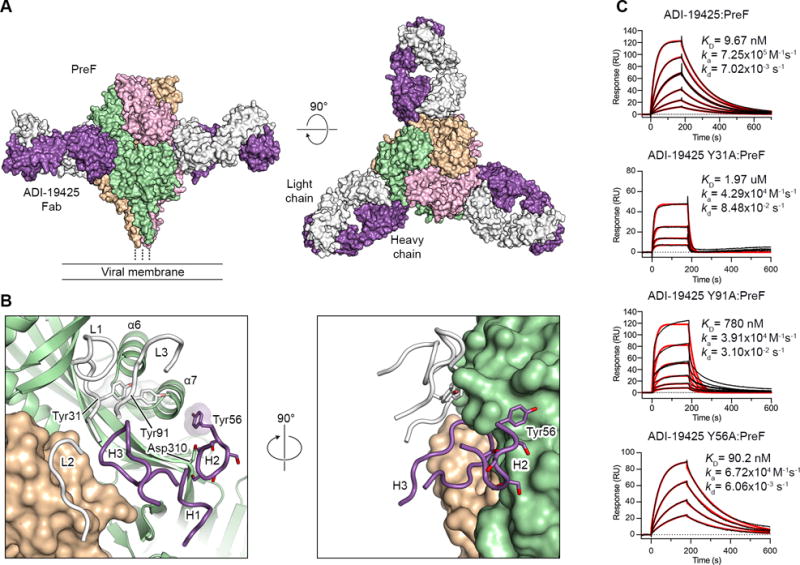
(A) Crystal structure of ADI-19425 Fab in complex with preF shown as molecular surfaces viewed along (left) and above (right) the viral membrane. The preF protomers are colored tan, pink and green, and the ADI-19425 heavy and light chains are colored purple and white, respectively.
(B) Magnified view of the antibody interface (left) and a 90° rotation about the vertical axis (right), colored as in (A). CDRs are shown as tubes and one RSV F protomer is shown as ribbons. Germline-encoded tyrosine and serine residues are shown as sticks with oxygen atoms colored red. Transparent molecular surfaces are shown for the three labeled tyrosine residues.
(C) Sensorgrams for the binding of ADI-19425 and the Y31A, Y91A and Y56A variants to preF measured using SPR. The data were double-reference subtracted (black) and fit to a 1:1 binding model (red). Results are representative of a single experiment.
