Abstract
Rationale: Obstructive sleep apnea (OSA) during REM sleep is a common disorder. Data on whether OSA that occurs predominantly during REM sleep is associated with health outcomes are limited.
Objectives: The present study examined the association between OSA during REM sleep and a composite cardiovascular endpoint in a community sample with and without prevalent cardiovascular disease.
Methods: Full-montage home polysomnography was conducted as part of the Sleep Heart Health Study. The study cohort was followed for an average of 9.5 years, during which time cardiovascular events were assessed. Only participants with a non-REM apnea–hypopnea index (AHI) of less than 5 events/h were included. A composite cardiovascular endpoint was determined as the occurrence of nonfatal or fatal events, including myocardial infarction, coronary artery revascularization, congestive heart failure, and stroke. Proportional hazards regression was used to derive the adjusted hazards ratios for the composite cardiovascular endpoint.
Measurements and Main Results: The sample consisted of 3,265 subjects with a non-REM AHI of less than 5.0 events/h. Using a REM AHI of less than 5.0 events/h as the reference group (n = 1,758), the adjusted hazards ratios for the composite cardiovascular endpoint in those with severe REM OSA (≥30 events/h; n = 180) was 1.35 (95% confidence interval, 0.98–1.85). Stratified analyses demonstrated that the association was most notable in those with prevalent cardiovascular disease and severe OSA during REM sleep with an adjusted hazards ratio of 2.56 (95% confidence interval, 1.46–4.47).
Conclusions: Severe OSA that occurs primarily during REM sleep is associated with higher incidence of a composite cardiovascular endpoint, but in only those with prevalent cardiovascular disease.
Keywords: sleep apnea, REM sleep, sleep-disordered breathing, cardiovascular disease, sleep heart
At a Glance Commentary
Scientific Knowledge on the Subject
Severe obstructive sleep apnea (OSA) that occurs exclusively during REM sleep is associated with recurrent cardiovascular events in those with prevalent cardiovascular disease.
What This Study Adds to the Field
This study seeks to determine whether OSA primarily during REM sleep increases the risk of cardiovascular disease. Severe OSA that is present only during REM sleep is associated with recurrent cardiovascular events in people who have prevalent cardiovascular disease.
It is well recognized that upper airway collapsibility and the severity of obstructive sleep apnea (OSA) can vary as a function of sleep stage (1). Rapid eye movement (REM) sleep, which typically accounts for 20–25% of total sleep time, is associated with distinct physiological alterations that influence upper airway function. For example, during REM sleep, there is a decrease in adrenergic and serotonergic control of the pharyngeal dilator muscles with accompanying cholinergic-mediated suppression of genioglossus activity. Consequently, there is an increased propensity for upper airway collapse (2, 3). In addition, OSA events during REM sleep are typically longer, more frequent, and are associated with greater oxyhemoglobin desaturation than events during non-REM (NREM) sleep. Furthermore, REM sleep is also associated with marked hemodynamic variability and an increase in sympathetic activity and myocardial demand (4–6).
Given the distinct physiologic characteristics of REM sleep, it is not surprising that OSA is common during REM sleep. In fact, 10–37% of patients referred for evaluation of OSA have apneas and/or hypopneas exclusively during REM sleep (7–11). Despite the frequent occurrence of OSA events during REM sleep, evidence of their health sequelae has been inconsistent. A few clinic-based studies have shown that OSA during REM sleep is associated with objective sleepiness (12) and that treatment may improve subjective sleepiness and quality of life (13). However, population-based studies have not confirmed these findings (14–16). Although limited, recent evidence on the cardiometabolic implications of OSA that occurs primarily during REM sleep is relatively consistent. REM sleep–related OSA has been independently associated with prevalent and incident hypertension (17, 18) as well as impairments in glucose metabolism (15, 19–21). Although hypertension and metabolic dysfunction can heighten the risk for cardiovascular disease, the potential influence of REM sleep–specific OSA on cardiovascular endpoints, such as myocardial infarction, stroke, coronary revascularization, and congestive heart failure, remains to be delineated. Without this evidence, the decision whether to treat patients with OSA only during REM sleep is challenging. Thus, the primary objective of the current investigation was to characterize the association between REM sleep–related OSA and a composite cardiovascular endpoint in a community-based cohort of middle-aged and older persons with and without cardiovascular disease.
Methods
Study Sample
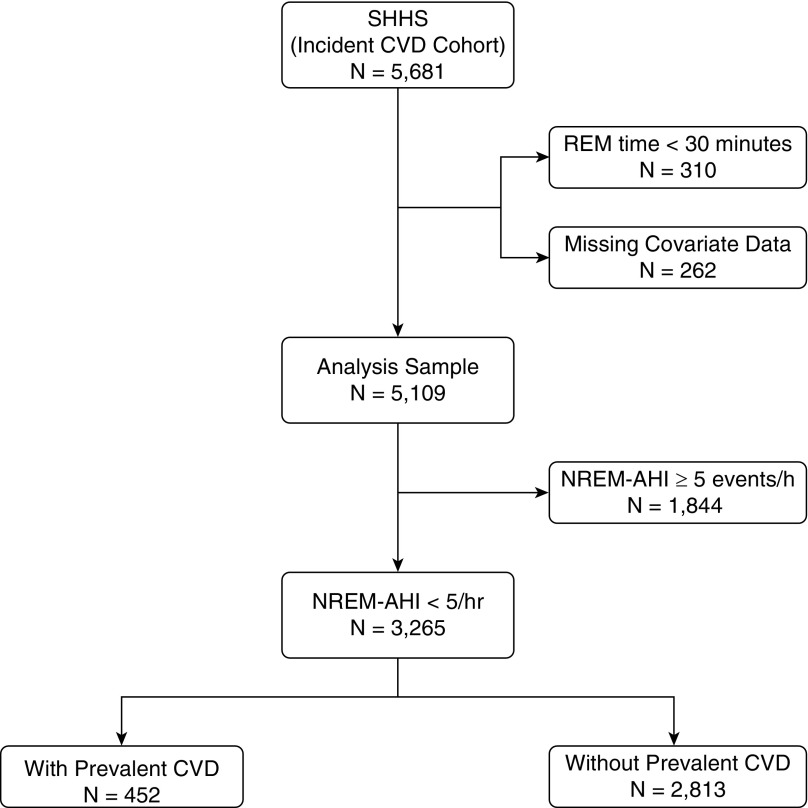
The Sleep Heart Health Study is a prospective cohort study of cardiovascular consequences of OSA. Details of the study design and cohort follow-up have been reported previously (22). Between 1995 and 1998, study participants were recruited from ongoing cohort studies, including the Framingham Offspring and Omni Study, the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Strong Heart Study, and the cohort studies of respiratory disease in Tucson. Participants were at least 40 years of age and were not on treatment for OSA with positive airway pressure, oral appliance, oxygen, or tracheostomy. All participants provided written consent, and the study protocol was approved by the institutional review board of each field site. Of the 5,681 participants who completed the baseline examination and had data on cardiovascular events, 3,265 participants without OSA during NREM sleep constituted the analysis sample for the current study.
Data Collection
All participants completed a baseline examination including a detailed health interview, unattended home polysomnogram, and measurements of blood pressure and anthopometry. Prevalent cardiovascular disease, determined by adjudicated surveillance data provided by the parent cohorts or by self-report at enrollment, included a history of physician-diagnosed myocardial infarction, stroke, coronary revascularization, and heart failure. Anthopometric measures, including weight, height, and waist girth, were obtained at the time of the sleep study by trained technicians. The sleep study was conducted using a portable monitor (P-Series; Compumedics). The following signals were recorded: C3/A1 and C4/A2 electroencephalograms, bilateral electrooculograms, a single bipolar electrocardiogram, a chin electromyogram, oxyhemoglobin saturation by pulse oximetry, chest and abdominal excursion by inductance plethysmography, airflow by an oronasal thermocouple, and body position by a mercury gauge. Details of polysomnographic equipment, hook-up procedures, failure rates, scoring, and quality assurance and control have been published previously (23). Apneas were identified if airflow was absent or nearly absent for at least 10 seconds. Apneas were further classified as obstructive if movement on either the chest or abdominal inductance channels was noted, or as central if no displacement was observed on both of these channels. Hypopneas were scored if there was at least 30% reduction in airflow or thoracoabdominal movement below baseline values for at least 10 seconds. The apnea–hypopnea index (AHI) was defined as the number of apneas and hypopneas, each associated with at least a 4% decrease in oxygen saturation per hour of sleep. An arousal index was derived as the total number of arousals per hour of sleep according to standard criteria (24). Only those participants with at least 30 minutes of REM sleep were included in the analysis to allow for a representative estimate of the REM AHI. Sensitivity analyses were also conducted requiring at least 15 minutes of REM sleep, which led to the inclusion of an additional 110 participants. These analyses showed no material differences in overall inferences, and thus to improve estimation of REM AHI, a threshold of 30 minutes was used.
During the follow-up period, the first occurrence of a cardiovascular event either nonfatal or fatal, the primary endpoint for this report, was identified and confirmed by the respective parent cohort using multiple concurrent approaches, including follow-up interviews, written annual questionnaires or telephone contacts with study subjects or next of kin, surveillance of local hospital records, and community obituaries. Using these methods, a total of 749 cardiovascular events was identified for the current study sample. Cardiovascular events of interest included the following: myocardial infarction (nonfatal or fatal); percutaneous coronary angioplasty; coronary artery bypass grafting; stroke (nonfatal or fatal); and occurrence of a heart failure episode. Incident hypertension was not included in the definition of the composite endpoint.
Statistical Analysis
Event rates were determined by dividing the number of events by the total number of person-years at risk. The REM AHI was categorized using commonly used clinical cutoff points: less than 5 (normal), 5.0–14.9 (mild disease), 15.0–29.9 (moderate disease), and 30.0 or more events/h (severe disease). Sensitivity analyses showed that inferences regarding the association between OSA severity and the cardiovascular endpoint were similar regardless of whether the REM AHI was categorized using clinical or quartile-based thresholds. Thus, the AHI was modeled as a categorical variable using the above clinical cut points to ease exposition. Kaplan-Meier plots were used to evaluate the association of REM AHI with the composite cardiovascular endpoint. Proportional hazards regression allowed for determination of unadjusted as well as adjusted relative hazard ratios for the occurrence of the composite cardiovascular endpoint. Covariates in the multivariable models included age, sex, race, smoking status (current, former, or never), and body mass index (BMI). Given the high degree of collinearity between BMI and waist circumference (r = 0.76), BMI was used as the covariate to account for obesity. To account for potential confounding, pre-existing medical conditions (e.g., prevalent hypertension and diabetes) at enrollment were also included as covariates. Inclusion of self-reported habitual sleep duration did not materially change the point estimates relating REM AHI to the composite cardiovascular endpoint, and thus it was not included in the final multivariable models for parsimony. To examine the influence of REM AHI on the composite cardiovascular endpoint, models were stratified by prevalent cardiovascular disease at the baseline visit. The SAS 9.0 software package (SAS Institute Inc.) was used for all analyses.
Results
The selection of the analytical sample from the larger cohort of the Sleep Heart Health Study is shown in Figure 1. The subset of those with an NREM AHI of less than 5 events/h included 452 and 2,813 participants with and without prevalent cardiovascular disease, respectively. Demographic and covariate data for the entire analysis sample, stratified by prevalent cardiovascular disease, are presented in Table 1. Participants with prevalent cardiovascular disease were older, had a higher BMI, and included more men than those without prevalent cardiovascular disease. In addition, the subgroup with prevalent cardiovascular disease included a greater proportion of non-white participants and former smokers, and had a higher prevalence of hypertension, diabetes, and REM sleep–related OSA. The distribution of REM AHI in the sample was as follows: 27.7% had mild disease (REM AHI: 5.0–14.9 events/h); 13.0% had moderate disease (REM AHI: 15.0–29.9 events/h); and 5.5% had severe disease (REM AHI ≥30 events/h). Not surprisingly, the proportion of those with moderate (31.9% vs. 17.5%; P < 0.001) and severe (14.4% vs. 8.6%; P = 0.013) REM sleep–related OSA was greater in those with than those without prevalent cardiovascular disease.
Figure 1.
The Sleep Heart Health Study (SHHS) sample. AHI = apnea–hypopnea index; CVD = cardiovascular disease; NREM = non-REM.
Table 1.
Baseline Characteristics in Participants with a Non-REM Apnea–Hypopnea Index of less than 5 Events per Hour by Prevalent Cardiovascular Disease
| All Participants (n = 3,265) | Participants without CVD (n = 2,813) | Participants with CVD (n = 452) | P Value* | |
|---|---|---|---|---|
| Age, yr, mean (SD) | 62.0 (10.7) | 61.0 (10.5) | 68.6 (9.5) | <0.001 |
| BMI, kg/m2, mean (SD) | 27.8 (5.0) | 27.7 (4.9) | 28.4 (5.2) | 0.004 |
| Waist girth, cm, mean (SD) | 95.5 (13.8) | 95.0 (13.8) | 98.6 (13.0) | <0.001 |
| Sex, % | ||||
| Women | 63.1 | 65.2 | 49.8 | <0.001 |
| Men | 36.9 | 34.8 | 50.2 | |
| Race, % | ||||
| White | 79.0 | 79.5 | 76.6 | <0.001 |
| African American | 5.2 | 4.9 | 6.8 | |
| Native American | 9.6 | 8.6 | 15.3 | |
| Other | 6.2 | 7.0 | 1.3 | |
| Smoking status, % | ||||
| Never | 47.1 | 48.3 | 40.0 | <0.001 |
| Former | 40.1 | 38.9 | 47.8 | |
| Current | 12.7 | 12.8 | 12.2 | |
| Hypertension, % | 44.7 | 39.6 | 76.1 | <0.001 |
| Diabetes, % | 8.9 | 7.1 | 19.7 | <0.001 |
| CVD, %† | 13.8 | 0.0 | 100.0 | — |
| REM AHI, events/h | ||||
| <5.0, % | 53.8 | 55.5 | 43.4 | <0.001 |
| 5.0–14.9, % | 27.7 | 27.5 | 29.0 | |
| 15.0–29.9, % | 13.0 | 11.8 | 20.3 | |
| ≥30.0, % | 5.5 | 5.2 | 7.3 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CVD = cardiovascular disease.
P value for comparisons of age, BMI, waist girth, sex, race, smoking status, hypertension, diabetes, and CVD between participants with and without CVD using χ2 and analysis of variance to compare categorical and continuous variables, respectively.
CVD defined as myocardial infarction, heart failure, stroke, or any coronary revascularization procedure.
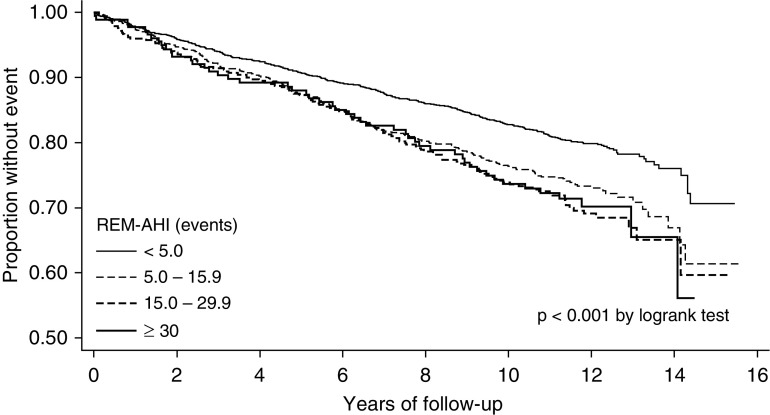
In total, the composite endpoint was observed in 749 participants, and the distribution of the first event was as follows: 418 were coronary artery disease events (e.g., myocardial infarction or coronary revascularization procedure); 233 were heart failure events; and 98 were strokes. Stratified analyses showed that the composite cardiovascular endpoint was observed in 53.5% and 18.0% of participants with and without prevalent cardiovascular disease, respectively (P < 0.001). Kaplan-Meier survival curves as a function of REM AHI for the sample stratified are shown in Figure 2. Moderate and severe OSA during REM sleep (REM AHI ≥15 events/h) were associated with a higher crude event rate for the composite cardiovascular endpoint. For example, as shown in Table 2, the unadjusted crude event rates for a REM AHI of 15.0–29.9 and 30 events/h or greater were 30.5 and 29.9 per 1,000 person-years, respectively, compared with 19.2 per 1,000 person-years in those with a REM AHI of less than 5 events/h. To determine whether REM AHI was independently associated with the occurrence of the composite cardiovascular endpoint, proportional hazards regression models were used. Nested models were used to assess incremental effects of various potentially confounding covariates on the association between the REM AHI and the composite cardiovascular endpoint. The base model included REM AHI as a categorical variable with adjustments for age, sex, race, BMI, and smoking status. The second model added prevalent diabetes mellitus and hypertension to the base model. Prevalent cardiovascular disease was added as a covariate in the model with all of the participants, but not in the models stratified by cardiovascular disease. Proportional hazards models using the nonstratified sample revealed that a REM AHI 30 events/h or greater was associated with a higher risk for the composite cardiovascular endpoint (Table 2). The association was marginal, but persisted after adjustments for prevalent comorbidities, such as hypertension, diabetes, and pre-existing cardiovascular disease (hazard ratio, 1.35; 95% confidence interval, 0.98–1.85). Stratified analyses showed a lack of an association between REM AHI and the composite cardiovascular endpoint in participants without prevalent cardiovascular disease. However, in participants with prevalent cardiovascular disease at baseline, there was an independent association between REM AHI and the composite cardiovascular endpoint. The adjusted hazard ratios for mild (REM AHI: 5.0–14.9 events/h), moderate (REM AHI: 15.0–29.9 events/h), and severe disease (REM AHI >30 events/h) were 1.20, 1.34, and 2.56, respectively (P = 0.005 for linear trend). Analyses were also conducted using the percent of REM sleep time below an oxygen saturation of 90% to quantify the degree of OSA. After adjusting for demographic covariates and prevalent comorbidity, an independent association was noted between the percent of REM sleep time below an oxygen saturation of 90% and the composite cardiovascular endpoint. The association seemed to be driven by those with the greatest percentage of REM sleep time spent below an oxygen saturation of 90% and prevalent cardiovascular disease (Table 3). Finally, no associations were noted between the frequency of REM sleep–related arousals and the composite cardiovascular endpoint (Table 4).
Figure 2.
Kaplan-Meier survival curves across categories of REM apnea–hypopnea index (AHI).
Table 2.
Adjusted Hazard Ratios for the Composite Cardiovascular Endpoint across Categories of REM Apnea–Hypopnea Index
| REM AHI (Events/h) | n | Person-Years | Events | Event Rate* | Model 1† Adjusted HR (95% CI) | Model 2‡ Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| All participants§ | ||||||
| <5.0 | 1,758 | 17,902 | 345 | 19.2 | 1.00 | 1.00 |
| 5.0–14.9 | 904 | 8,627 | 231 | 26.8 | 1.22 (1.03–1.45) | 1.24 (1.05–1.47) |
| 15.0–29.9 | 423 | 4,037 | 123 | 30.5 | 1.17 (0.95–1.46) | 1.14 (0.91–1.41) |
| ≥30.0 | 180 | 1,672 | 50 | 29.9 | 1.29 (0.94–1.77) | 1.35 (0.98–1.85) |
| Without prevalent CVD | ||||||
| <5.0 | 1,562 | 16,450 | 242 | 14.7 | 1.00 | 1.00 |
| 5.0–14.9 | 773 | 7,701 | 163 | 21.1 | 1.25 (1.02–1.55) | 1.25 (1.02–1.53) |
| 15.0–29.9 | 331 | 3,510 | 71 | 20.8 | 1.02 (0.77–1.34) | 1.02 (0.78–1.35) |
| ≥30.0 | 147 | 1,467 | 31 | 21.1 | 1.09 (0.73–1.61) | 1.06 (0.72–1.58) |
| With prevalent CVD | ||||||
| <5.0 | 196 | 1,452 | 103 | 70.9 | 1.00 | 1.00 |
| 5.0–14.9 | 131 | 923 | 68 | 73.5 | 1.17 (0.85–1.60) | 1.20 (0.88–1.64) |
| 15.0–29.9 | 92 | 626 | 52 | 83.0 | 1.33 (0.93–1.90) | 1.34 (0.94–1.91) |
| ≥30.0 | 33 | 204 | 19 | 92.9 | 2.41 (1.39–4.17) | 2.56 (1.46–4.47) |
Definition of abbreviations: AHI = apnea–hypopnea index; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio.
Crude event rate per 1,000 person-years.
Model 1: adjusted for age (continuous), sex, race, body mass index (continuous), and smoking status (never, former, current).
Model 2: adjusted for covariates of model 1 and prevalent hypertension and diabetes.
For models with all participants, prevalent CVD was included as a covariate in model 2.
Table 3.
Adjusted Hazard Ratios for the Composite Cardiovascular Endpoint across Quartiles of REM Sleep Time Percentage below 90% in Participants with a NREM-AHI less than 5 Events per Hour
| % REM Sleep Time with SpO2 <90% | n | Person-Years | Events | Event Rate* | Model 1† Adjusted HR (95% CI) | Model 2‡ Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| All participants§ | ||||||
| Q1–Q2 (<0.03) | 1,615 | 16,627 | 308 | 18.5 | 1.00 | 1.00 |
| Q3 (0.03–1.42) | 807 | 7,888 | 178 | 22.6 | 1.02 (0.85–1.24) | 1.02 (0.84–1.22) |
| Q4 (>1.42) | 807 | 7,345 | 255 | 34.7 | 1.24 (1.04–1.49) | 1.21 (1.02–1.45) |
| Without prevalent CVD | ||||||
| Q1–Q2 (<0.01) | 1,399 | 14,867 | 217 | 14.6 | 1.00 | 1.00 |
| Q3 (0.01–1.13) | 690 | 7,093 | 113 | 15.9 | 0.95 (0.75–1.20) | 0.97 (0.77–1.23) |
| Q4 (>1.13) | 695 | 6,758 | 170 | 25.1 | 1.13 (0.91–1.40) | 1.11 (0.90–1.38) |
| With prevalent CVD | ||||||
| Q1–Q2 (<0.33) | 223 | 1,746 | 114 | 70.0 | 1.00 | 1.00 |
| Q3 (0.34–4.04) | 111 | 783 | 59 | 79.0 | 1.23 (0.89–1.69) | 1.18 (0.88–1.64) |
| Q4 (>4.04) | 111 | 799 | 68 | 88.9 | 1.30 (0.94–1.79) | 1.36 (0.99–1.89) |
Definition of abbreviations: AHI = apena–hypopnea index; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; NREM = non-REM; Q = quartile; SpO2 = oxygen saturation as measured by pulse oximetry.
Crude event rate per 1,000 person-years.
Model 1: adjusted for age (continuous), sex, race, body mass index (continuous), and smoking status (never, former, current).
Model 2: adjusted for covariates of model 1 and prevalent hypertension and diabetes.
For models with all participants, prevalent CVD was included as a covariate in model 2.
Table 4.
Adjusted Hazard Ratios for the Composite Cardiovascular Endpoint across Quartiles of Arousal Frequency during REM Sleep in Participants with a NREM-AHI less than 5 Events per Hour
| % REM Sleep Time with SpO2 <90% | n | Person-Years | Events | Event Rate* | Model 1† Adjusted HR (95% CI) | Model 2‡ Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| All participants§ | ||||||
| Q1(<6.9) | 817 | 7,868 | 209 | 26.6 | 1.00 | 1.00 |
| Q2 (6.9–11.3) | 816 | 8,002 | 180 | 22.5 | 0.90 (0.74–1.10) | 0.85 (0.70–1.05) |
| Q3 (11.4–17.8) | 816 | 8,200 | 175 | 21.3 | 0.84 (0.69–1.03) | 0.88 (0.71–1.07) |
| Q4 (≥17.9) | 816 | 8,168 | 185 | 22.7 | 0.85 (0.70–1.04) | 0.88 (0.72–1.07) |
| Without prevalent CVD | ||||||
| Q1(<6.9) | 706 | 7,102 | 147 | 20.7 | 1.00 | 1.00 |
| Q2 (6.9–11.4) | 708 | 7,255 | 120 | 16.5 | 0.88 (0.69–1.12) | 0.85 (0.67–1.08) |
| Q3 (11.5–17.9) | 696 | 7,305 | 114 | 15.6 | 0.81 (0.64–1.04) | 0.85 (0.67–1.08) |
| Q4 (≥18.0) | 703 | 7,367 | 126 | 17.1 | 0.85 (0.66–1.08) | 0.87 (0.69–1.11) |
| With prevalent CVD | ||||||
| Q1(<6.8) | 113 | 810 | 61 | 75.9 | 1.00 | 1.00 |
| Q2 (6.9–11.1) | 113 | 803 | 59 | 73.4 | 0.96 (0.67–1.37) | 0.92 (0.64–1.32) |
| Q3 (11.2–17.5) | 114 | 811 | 61 | 75.2 | 1.00 (0.69–1.43) | 1.01 (0.70–1.46) |
| Q4 (≥17.6) | 112 | 783 | 61 | 77.9 | 1.05 (0.73–1.51) | 1.10 (0.76–1.57) |
For definition of abbreviations, see Table 3.
Crude event rate per 1,000 person-years.
Model 1: adjusted for age (continuous), sex, race, body mass index (continuous), and smoking status (never, former, current).
Model 2: adjusted for covariates of model 1 and prevalent hypertension and diabetes.
For models with all participants, prevalent CVD was also included as a covariate in model 2.
Discussion
The results of the current study demonstrate several relevant findings about the association between REM sleep–specific OSA and cardiovascular disease. First, OSA occurring exclusively during REM sleep is associated with recurrence of cardiovascular disease in a community-based cohort of middle- to older-aged adults after adjustment for demographic characteristics, smoking status, hypertension, and diabetes. Second, the noted association was driven primarily by those individuals with underlying prevalent cardiovascular disease. Next, analyses of physiological indices of OSA demonstrated that severity of REM sleep–related hypoxemia, defined as the percent of REM time below an oxygen saturation of 90%, was marginally associated with the composite cardiovascular endpoint. Finally, no associations were identified between the frequency of arousals during REM sleep and cardiovascular disease.
OSA during REM sleep has become a topic of significant clinical debate and research over the last decade. Results from studies examining objective and subjective sleepiness, as well as quality of life, have largely been conflicting, with some clinic-based studies demonstrating an association between and OSA during REM sleep, objective sleepiness (12), and health-related quality-of-life surveys (13). Population-based studies, however, have not replicated such associations (13–15). Interestingly, research on the influence of OSA during REM sleep and cardiometabolic outcomes, specifically hypertension and alterations in glucose metabolism, has yielded somewhat more consistent results (19, 20). For example, cross-sectional analyses of two distinct patient samples with type 2 diabetes have shown that glycemic control, as assessed by glycosylated hemoglobin, is associated with the REM AHI, but not the NREM AHI (20). Previous analyses of the Sleep Heart Health Study data have also shown that insulin resistance, as determined by the homeostatic model assessment of insulin resistance, is associated with the severity of OSA during REM sleep (19). When hypertension is considered as an outcome, the evidence, which is derived primarily from community-based studies, is relatively concordant. Data from the Wisconsin Sleep Cohort Study have shown an association between severe REM sleep–related OSA (REM AHI ≥15 events/h) and prevalent as well incident hypertension, particularly when ambulatory blood pressure monitoring is used (18). Incident nocturnal nondipping of both systolic and diastolic blood pressures on ambulatory blood pressure monitoring were noted in those with severe OSA during REM sleep (25). Similar results linking REM AHI to prevalent and recent-onset hypertension were also reported in a community-based cohort of over 700 men (17). The current investigation extends the limited body of evidence and provides unique insights on the cardiometabolic consequences of REM sleep–specific OSA by demonstrating an independent association with the development of downstream, clinically relevant cardiovascular endpoints in susceptible individuals (i.e., those with prevalent cardiovascular disease). Indeed, prior work on OSA occurring both during NREM and REM sleep has also supported an association with subsequent major cardiovascular and cerebrovascular events in those with underlying cardiovascular disease (26–33). However, the findings from the present study are distinct in that they indicate that, despite the limited duration and exposure time seen with OSA confined to REM sleep, an independent association persists with recurrent cardiovascular events. The noted association could be driven by OSA-associated nocturnal hypoxemia.
Given the preponderance of evidence demonstrating an association between OSA and cardiovascular outcomes in those with prevalent cardiovascular disease, it is surprising that the literature on the effects of continuous positive airway pressure (CPAP) has not shown a consistent beneficial effect. For example, three separate observational studies (34–36) and one randomized trial (37) have previously demonstrated that CPAP use results in a decline in either cardiac deaths (34), recurrent myocardial infarction (35), revascularization procedures (35, 36), mean arterial blood pressure (37), or inflammatory biomarkers, such as C-reactive protein (37, 38), in those with sleep stage–nonspecific OSA (occurring both during NREM and REM) and prevalent cardiovascular disease. However, the SAVE (Sleep Apnea Cardiovascular Endpoints) trial, a large, multicenter, randomized, controlled trial, examining the effects of CPAP therapy on secondary prevention of incident cardiovascular events, failed to find a favorable effect (39). There are several plausible reasons explicating the lack of a therapeutic benefit in this latter study. First, the overall average CPAP adherence of 3.3 h/night noted in the SAVE trial is a possible limitation. Other studies have uncovered a more favorable treatment effect on cardiovascular outcomes when CPAP is used for 4 h/night or longer (40, 41). However, subgroup analysis of the participants in the highest quintile of CPAP use (>5.6 h/night) showed no favorable impact on recurrent cardiovascular endpoints. Second, when subjective sleepiness determined by the Epworth Sleepiness Scale (ESS) is considered, the existing evidence suggests that CPAP therapy does not positively impact cardiovascular outcomes (42, 43) or incident hypertension (43) in patients who do not report excessive daytime sleepiness (ESS <10). Participants in the SAVE trial did not, on average, exhibit daytime sleepiness, with both the treatment and control arms having baseline ESS scores of 7.3 and 7.5, respectively. Thus, it is possible that daytime sleepiness may have a differential influence on the association between CPAP therapy and cardiovascular outcomes. Third, it is conceivable that exposure to chronic intermittent hypoxemia seen with OSA results in pathophysiological derangements, such as inflammation, oxidative stress, and endothelial dysfunction (44), that are irreversible in the setting of cardiovascular disease. Consequently, there may not be a notable response to CPAP therapy. In fact, experimental work by Polak and colleages (45) has shown that the metabolic derangements associated with intermittent hypoxemia were not reversed after cessation of the hypoxic exposure and were associated with persistent disruption of glucose homeostasis. A similar effect may be seen in those with cardiovascular disease and concurrent OSA. Finally, REM sleep–specific OSA may just be a marker and reflect greater underlying cardiovascular risk.
There are several strengths and limitations of the current investigation that warrant discussion. The large sample size from a community-based population makes the findings more generalizable and germane. The cohort was representative of both sexes, and included the full spectrum of REM-related OSA severity. Limitations include the potential of residual confounding by very mild OSA during NREM sleep and the small sample size in those with both prevalent cardiovascular disease and severe REM sleep–specific OSA. Despite these limitations, the results herein stand to fill existing knowledge gaps on the association between OSA occurring exclusively during REM sleep and incidence of cardiovascular events. In conclusion, the current investigation shows an independent association between REM sleep–specific OSA and recurrent cardiovascular events in those with prevalent cardiovascular disease. Future work on this topic should take into consideration concepts such as duration and reversibility of disease and potential effect modification by other comorbid conditions.
Footnotes
Supported by NIH grants K23-HL118414 (R.N.A.) and R01-HL075078 (N.M.P.). The Sleep Heart Health Study was supported by the NHLBI through the following cooperative agreements: U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL63463 (Case Western Reserve University), U01HL53937 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL63429 (Missouri Breaks Research), and U01HL53931 (New York University).
Author Contributions: study design and collection of data—D.J.G. and N.M.P.; analysis of data—R.N.A., C.C., J.S.K., and N.M.P.; interpretation of findings—R.N.A., C.C., J.S.K., D.J.G., and N.M.P.; and preparation of manuscript—R.N.A., C.C., J.S.K., D.J.G., and N.M.P.
Originally Published in Press as DOI: 10.1164/rccm.201706-1112OC on November 7, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Carberry JC, Hensen H, Fisher LP, Saboisky JP, Butler JE, Gandevia SC, et al. Mechanisms contributing to the response of upper-airway muscles to changes in airway pressure. J Appl Physiol. 1985;2015:1221–1228. doi: 10.1152/japplphysiol.01103.2014. [DOI] [PubMed] [Google Scholar]
- 2.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 3.McSharry DG, Saboisky JP, Deyoung P, Jordan AS, Trinder J, Smales E, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37:561–569. doi: 10.5665/sleep.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, et al. Modulations of heart rate, ECG, and cardio-respiratory coupling observed in polysomnography. Front Physiol. 2016;7:460. doi: 10.3389/fphys.2016.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–526. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 8.Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement–related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–3357. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 9.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–264. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 10.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement–related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–1161. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resta O, Carpanano GE, Lacedonia D, Di Gioia G, Giliberti T, Stefano A, et al. Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA) Respir Med. 2005;99:91–96. doi: 10.1016/j.rmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement–specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–169. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- 13.Su CS, Liu KT, Panjapornpon K, Andrews N, Foldvary-Schaefer N. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med. 2012;8:243–247. doi: 10.5664/jcsm.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chami HA, Baldwin CM, Silverman A, Zhang Y, Rapoport D, Punjabi NM, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep–disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan A, Harrison SL, Kezirian EJ, Ancoli-Israel S, O’Hearn D, Orwoll E, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) Sleep Study. J Clin Sleep Med. 2013;9:191–198. doi: 10.5664/jcsm.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–314. [PubMed] [Google Scholar]
- 17.Appleton SL, Vakulin A, Martin SA, Lang CJ, Wittert GA, Taylor AW, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150:495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension: results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chami HA, Gottlieb DJ, Redline S, Punjabi NM. Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am J Respir Crit Care Med. 2015;192:1118–1126. doi: 10.1164/rccm.201501-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37:355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, et al. Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med. 2016;194:516–519. doi: 10.1164/rccm.201602-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 23.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 24.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70:1062–1069. doi: 10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leão S, Conde B, Fontes P, Calvo T, Afonso A, Moreira I. Effect of obstructive sleep apnea in acute coronary syndrome. Am J Cardiol. 2016;117:1084–1087. doi: 10.1016/j.amjcard.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–621. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 29.Loo G, Tan AY, Koo CY, Tai BC, Richards M, Lee CH. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014;15:631–636. doi: 10.1016/j.sleep.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Ludka O, Stepanova R, Vyskocilova M, Galkova L, Mikolaskova M, Belehrad M, et al. Sleep apnea prevalence in acute myocardial infarction—the Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) Study. Int J Cardiol. 2014;176:13–19. doi: 10.1016/j.ijcard.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima H, Kurobe M, Minami K, Furudono S, Uchida Y, Amenomori K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2015;4:75–84. doi: 10.1177/2048872614530865. [DOI] [PubMed] [Google Scholar]
- 33.Steiner S, Schueller PO, Hennersdorf MG, Behrendt D, Strauer BE. Impact of obstructive sleep apnea on the occurrence of restenosis after elective percutaneous coronary intervention in ischemic heart disease. Respir Res. 2008;9:50. doi: 10.1186/1465-9921-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–1314. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Rio F, Alonso-Fernández A, Armada E, Mediano O, Lores V, Rojo B, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168:1328–1335. doi: 10.1016/j.ijcard.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Lv S, Yu X, Yao L, Mokhlesi B, Wei Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest. 2015;147:708–718. doi: 10.1378/chest.14-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng S, Fang L, Wang CQ, Wang LS, Chen MT, Huang XH. Impact of obstructive sleep apnoea on clinical characteristics and outcomes in patients with acute coronary syndrome following percutaneous coronary intervention. J Int Med Res. 2009;37:1343–1353. doi: 10.1177/147323000903700509. [DOI] [PubMed] [Google Scholar]
- 39.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 40.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 41.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 42.Barbé F, Mayoralas LR, Duran J, Masa JF, Maimó A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med. 2001;134:1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 43.Phillips B, Shafazand S. CPAP and hypertension in nonsleepy patients. J Clin Sleep Med. 2013;9:181–182. doi: 10.5664/jcsm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polak J, Shimoda LA, Drager LF, Undem C, McHugh H, Polotsky VY, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:1483–1490; 1490A–1490B. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]