Table 1. Different hydroxylation approaches12, c .
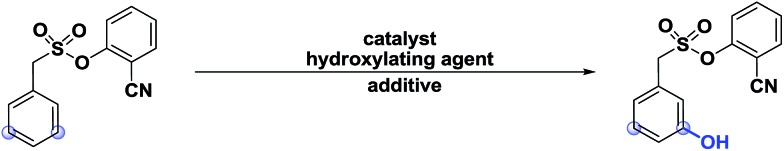
| |||
| Catalyst a | –OH source | Yield | |
| 1 | PdCl2/Pd(OAc)2 | TBHP (4 eq.) | 0 |
| 2 | PdCl2 | H2O2 (4 eq.) | 0 |
| 3 | PdCl2 | NHPI (2 eq.) | 0 |
| 4 | Cu(OAc)2 | (PhCO)2O (2 eq.), HFIP (1 mL) | 0 |
| 5 | PdCl2/Pd(OAc)2 | TEMPO (2 eq.) | 0 |
| 6 | Cu(OAc)2 | TBAI(2 eq.), Ag2CO3(2 eq.) | 0 |
| 7 | PdCl2 | K2S2O8 (2 eq.), CF3COOH (0.5 mL) | 0 |
| 8 | Pd(OAc)2 | Na2S2O8 (2 eq.); dioxane (1 mL) | 0 |
| 9 b | Pd(OAc)2 | PhI(TFA)2(4 eq.), (CF3CO)2O | 11 |
| 10 | Pd(OAc)2 | PhI(TFA)2 (4 eq.), HFIP (1 mL) | 78 |
aCatalyst loading 10 mol%.
b0.5 mL of (CF3CO)2O added.
c70 °C was maintained for all the reactions; all the reactions were performed on a 0.2 mmol scale.
