Abstract
Objectives
Lower vitamin D status has been associated with adiposity in children through adults. However, the evidence of the impact of maternal vitamin-D status during pregnancy on offspring’s adiposity is mixed. The objective of this study was to examine the associations between maternal vitamin-D [25(OH)D] status at mid-gestation and neonatal abdominal adipose tissue (AAT) compartments, particularly the deep subcutaneous adipose tissue linked with metabolic risk.
Methods
Participants (N = 292) were Asian mother-neonate pairs from the mother-offspring cohort, Growing Up in Singapore Towards healthy Outcomes. Neonates born at ≥34 weeks gestation with birth weight ≥2000 g had magnetic resonance imaging (MRI) within 2-weeks post-delivery. Maternal plasma glucose using an oral glucose tolerance test and 25(OH)D concentrations were measured. 25(OH)D status was categorized into inadequate (≤75.0 nmol/L) and sufficient (>75.0 nmol/L) groups. Neonatal AAT was classified into superficial (sSAT), deep subcutaneous (dSAT), and internal (IAT) adipose tissue compartments.
Results
Inverse linear correlations were observed between maternal 25(OH)D and both sSAT (r = −0.190, P = 0.001) and dSAT (r = −0.206, P < 0.001). Each 1 nmol/L increase in 25(OH)D was significantly associated with reductions in sSAT (β = −0.14 (95% CI: −0.24, −0.04) ml, P = 0.006) and dSAT (β = −0.04 (−0.06, −0.01) ml, P = 0.006). Compared to neonates of mothers with 25(OH)D sufficiency, neonates with maternal 25(OH)D inadequacy had higher sSAT (7.3 (2.1, 12.4) ml, P = 0.006), and dSAT (2.0 (0.6, 3.4) ml, P = 0.005) volumes, despite similar birth weight. In the subset of mothers without gestational diabetes, neonatal dSAT was also greater (1.7 (0.3, 3.1) ml, P = 0.019) in neonates with maternal 25(OH)-inadequacy. The associations with sSAT and dSAT persisted even after accounting for maternal glycemia (fasting and 2-h plasma glucose).
Conclusions
Neonates of Asian mothers with mid-gestation 25(OH)D inadequacy have a higher abdominal subcutaneous adipose tissue volume, especially dSAT (which is metabolically similar to visceral adipose tissue in adults), even after accounting for maternal glucose levels in pregnancy.
Subject terms: Translational research, Epidemiology
Introduction
Obesity in childhood leads to a wide range of long-term health complications. Without intervention, obese infants, and young children are more likely to remain obese in adolescent and adulthood, and at increased risk of metabolic diseases later in life [1]. The developmental origins of health and disease (DOHaD) concept posits an important role for early life environmental factors in programming offspring’s metabolic susceptibility over the lifecourse [2]. The in utero environment may partly influence offspring’s health by altering materno-fetal transfer of glucose and fatty acids leading to fetal fat deposition [3]. Therefore, body composition at birth may be an indicator of the in utero environment during development, and may form the basis for future cardio-metabolic risk [4].
With the recognition of developmental influences on obesity risk and the increasing prevalence of childhood obesity, research has focused on identifying potentially modifiable early life risk factors as a basis for timely interventions. Vitamin D has been increasingly studied for its potential influence on a range of chronic diseases including type 2 diabetes mellitus (T2DM) and cardiovascular diseases beyond its known role in bone mineral metabolism [5]. Vitamin D is required for insulin secretion by pancreatic β-cells and thus may also have a role in insulin sensitivity [6]. Subjects with hypovitaminosis D had higher risk of insulin resistance and the metabolic syndrome [7]. Similarly, low-serum 25-hydroxy vitamin D [25(OH)D] is associated with obesity, insulin resistance and β-cell dysfunction in children and adolescents [8]. These findings suggest a potential role for 25(OH)D in maintaining glucose metabolism and pancreatic β-cell function during pregnancy.
25(OH)D deficiency is common in pregnant women worldwide [9] including Asian women [10]. The fetus does not produce vitamin D and thus depends on maternal vitamin D supply through the placenta. Maternal 25(OH)D status during pregnancy is strongly correlated with the offspring’s 25(OH)D status [11] and has been linked with the offspring’s growth and body composition [12–14]. Although growing evidence suggests a role of maternal vitamin D on offspring growth and adiposity, findings are mainly from a small number of studies in western populations and inconsistent. In addition, “adiposity” in most of those studies was measured by body mass index (BMI), which reflects both fat mass and fat free mass and does not separate abdominal and non-abdominal adiposity. Abdominal adiposity is especially relevant in Asians who are at higher metabolic risk than the western population, even at lower or similar BMI or waist circumference [15, 16]. Therefore, we hypothesized that maternal vitamin D inadequacy during pregnancy would be associated with higher abdominal adiposity in the newborn Asian infants. This study aimed to focus on the association of 25(OH)D status of Asian mothers at mid-gestation with neonatal abdominal adiposity measured by abdominal adipose tissue (AAT) compartment volumes using magnetic resonance imaging (MRI).
Materials/subjects and methods
Study design
Participants were from a prospective birth cohort study in Singapore, Growing Up in Singapore Towards healthy Outcomes (GUSTO) [17]. A total of 1247 pregnant women in their first trimester were recruited between June 2009 and September 2010 at the two major public maternity units; KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH). Details of the inclusion and exclusion criteria were discussed earlier [17]. This study was approved by the Domain Specific Review Board of National Health Care group and Centralized Institutional Review Board, SingHealth. Written informed consent was given by all participants.
Subjects
A total of 1115 mothers who attended the 32–34 week antenatal ultrasound scan were approached for MRI of their newborns. A total of 478 (43%) mothers signed consent to neonatal magnetic resonance imaging (MRI) of their neonates. Neonates born earlier than late preterm i.e., <34 weeks gestational age with birth weight <2000 g were excluded from this study as they were more prone to complications such as hypothermia. Neonates included in this study were not on special neonatal care or neonatal intensive care unit at the time of MRI. MRI was performed preferably within 2 weeks after birth. 416 neonates were eligible; 379 neonates successfully competed MRI and 37 neonates dropped out. A total of 46 datasets did not pass initial quality control for image analysis [18]. Neonates of mothers who did not have 25(OH)D at 26–28 weeks gestation (N = 41) were also excluded. A total of 292 mother-neonate pairs remained for this analysis: 161 male (55.1%), 131 female (44.9%); 131 Chinese (44.9%), 115 Malay (39.4%), and 46 Indian (15.7%). We considered this study as an exploratory comparison and no a priori sample size calculations were performed. However, from the data we observed in vitamin D sufficiency (N = 156) and inadequacy (N = 136) groups, we are able to show a difference of 0.35 Cohen’s effect with 80% power and two sided 5% type 1 error.
In Supplemental Table 1, we compare the neonates included (N = 292) and those not included (N = 801) in this study. A total of 823 (1115–292) mothers were approached in pregnancy about taking part in the MRI sub-study but were not included in the final analysis, of whom 22 were born <34 gestational weeks with birth weight <2000 g. Therefore, the final non-participant group comprises 803 neonates. Participating neonates had mothers who were younger, more likely to be of Malay ethnicity and less likely to have higher than a secondary education than those non-participating infants. Mothers of participating neonates also had marginally higher FPG and 2-h OGTT glucose and lower 25(OH)D levels. Compared with non-participants, participating neonates had a similar mean birth weight and similar proportions of male and female neonates, but a marginally lower gestational age at delivery (38.7 vs 38.9 weeks) (Supplemental Table 1).
Maternal and infant characteristics
Demographic data, lifestyle, obstetric, and medical history of mothers were collected at 11–12 and 26–28 weeks during antenatal visits using interviewer administered questionnaires. Birth weights measured by midwives at KKH and NUH were obtained from hospital medical records.
Maternal biosample collection for glucose and vitamin D assessment
Blood samples of mothers were collected after at least 8–10 h of fasting, at 26–28 weeks antenatal clinic visit, processed within 4 h of collection and stored at −80 °C for subsequent analysis.
Plasma samples for 25(OH) D2 and D3 were extracted using vortex-mixing with hexane (Chromonorm). The hexane layer was then evaporated to dryness and reconstituted using a methanol-water (70:30 by volume) mixture (HyperSolv). Analysis of 25(OH)D concentration was performed using Applied Biosystems (USA) liquid chromatography-tandem mass spectrometry with its analyst software version. 1.3. Chromatographic separation was carried out with a BDS C8 reversed-phase column (ThermoHypersil) [19]. The intra- and inter-assay coefficient of variations for 25(OH)D2 and 25(OH)D3 were ≤10.3%, and the detection limit was <4 nmol/L for both metabolites. Maternal 25(OH)D status i.e., combined 25 (OH)D2 and 25(OH)D3 was categorized into inadequacy ≤75.0 nmol/L vs. sufficiency: >75.0 nmol/L based on recommendations by the Endocrine Society Clinical Practice Guidelines [20].
Mothers who attended 26–28 week antenatal visit after overnight fasting underwent 75-g OGTT. Blood samples were collected at baseline for fasting plasma glucose (FPG) and following a 75-g glucose challenge for 2-h plasma glucose (2hPG). Glucose levels were measured by colorimetry [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics) and Beckman LX20 Pro analyzer (Beckman Coulter)]. Gestational diabetes mellitus (GDM) was diagnosed using 1999 World Health Organization standard criteria: ≥7.0 or ≥7.8 mmol/l for FPG or 2hPG, respectively [21]. Pregnant women diagnosed with GDM were managed according to the clinical protocols at KKH and NUH.
Quantification of abdominal adipose tissue compartments
MRI was performed using GE Signa HDxt 1.5 tesla magnetic resonance scanner (GE Healthcare, Wauwatosa, Wisconsin, USA). The AAT from MRI images were categorized into superficial subcutaneous (sSAT), deep subcutaneous (dSAT), and internal (IAT) compartments (Fig. 1). sSAT had a clear anatomical outline following the contours of the axial abdominal images. dSAT was located mainly posteriorly and was distinctly separated from sSAT by a fascial plane. IAT was the internal fat including VAT i.e., amount of fat around the internal organs of abdominal cavity, inter-muscular, para-vertebral, and intra-spinal fat within the abdominal region.
Fig. 1.
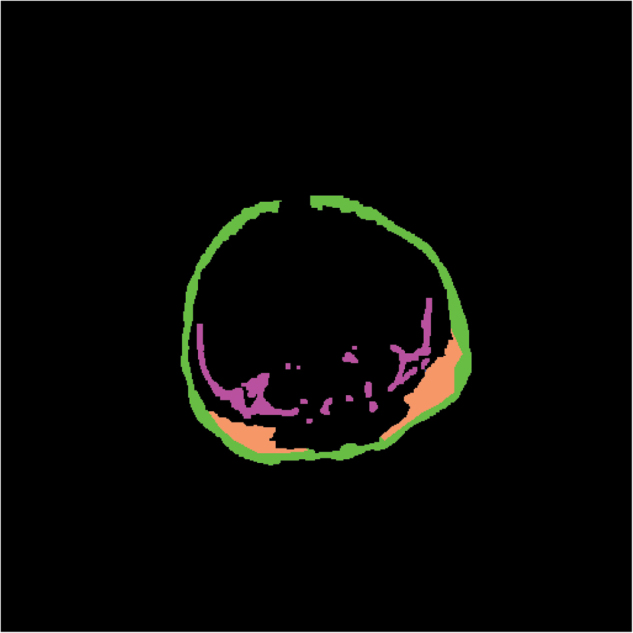
MRI image of the segmented abdominal adipose tissue compartments. Each compartment is filled with different colors: green denotes superficial subcutaneous adipose tissue, orange denotes the right and left deep subcutaneous adipose tissue; magenta denotes the internal adipose tissue
AAT compartments were segmented using semi-automated approach; with initial automated segmentation by in-house segmentation algorithm using MATLAB 7.13; The MathWorks Inc., Natick, Massachusetts, USA and followed by manual optimization by 2 trained analysts who were blinded to all subject information. The mean inter-observer coefficients of variations (%) were 1.6% for sSAT, 3.2% for dSAT, and 2.1% for IAT. The mean intra-observer coefficients of variations (%) were 0.9%, 2.1% and 4.0% for sSAT, dSAT, and IAT, respectively. Total AAT volume for each compartment was derived from the sum of its volumes in each slice, from the level of dome of diaphragm to the superior aspect of the sacrum [18].
Statistical analysis
As the neonatal AAT compartment volumes were normally distributed (Supplemental Fig. 1), multivariable regression models were used to assess the overall associations between maternal 25(OH)D (either as continuous or dichotomous categorical variables) and neonatal AAT compartment volumes. Covariates adjusted for included ethnicity, sex, age on MRI day, gestational age, maternal age, maternal education, maternal pre-pregnancy BMI, and parity. We did not adjust for birth weight, as it may be on the causal pathway between maternal 25(OH)D status and neonatal AAT compartment volumes. In addition, we have shown that maternal 25(OH)D status was not associated with birth weight in GUSTO cohort [22]. Type of neonatal feeding (breastfeeding or formula feeding) was not included in the multivariable models, as the types of neonatal feeding within 2 weeks of delivery should not have a substantial influence on neonatal adiposity.
25(OH)D and its deficiency were related to glucose homeostasis [23, 24], which may in turn influence on their offspring’s adiposity. In our study, maternal 25(OH)D and glucose levels were measured using blood samples collected at the same time, and we are therefore unable to analyze their temporal relationship [24]. Nonetheless, to examine whether the 25 (OH)D-neonates adiposity association was mediated by concurrently measured glycemia, we performed a secondary analysis by further adjusting for maternal glycemia, both FPG and 2HrPG, in multivariable regression analyses. We performed these analyses both in the overall group of 292 neonates and as a sensitivity analysis in the subset (N = 237) of mothers without GDM (non-GDM). Statistical analyses were performed using SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY).
Results
Table 1 summarizes the baseline characteristics of study participants (N = 292). A total 136 (46.6%) of 292 mothers were classified as having 25(OH)D inadequacy although 183 (62.7%) reported taking supplements containing vitamin D at the time of blood collection. 25(OH)D inadequacy was higher in Malay (55.9%) than Chinese (25.7%) and Indian mothers (18.4%) (P < 0.001). Mothers with 25(OH)D inadequacy were younger, mean ± SD: 29 ± 5 vs 30 ± 5 years (P = 0.010) and had a higher pre-pregnancy BMI 23.8 ± 5.1 vs 22.4 ± 4.6 kg/m2 (P = 0.019) than those who had sufficient 25(OH)D. Mean FPG and 2hPG levels at 26–28 weeks of gestation were similar in the 25(OH)D sufficient and inadequate groups. Neonates between 2 groups had similar mean birth weight and weight on MRI day, and were born at similar gestational weeks. However, neonates with maternal 25(OH)D inadequacy had greater sSAT and dSAT compared to those with maternal 25(OH) sufficiency; 81.3 ± 22.5 vs 74.7 ± 21.0 ml, P = 0.010 for sSAT and 14.3 ± 5.8 vs 12.2 ± 5.2 ml, P = 0.002 for dSAT.
Table 1.
Maternal and neonatal baseline characteristics by maternal plasma 25(OH)D levels among 292 participants in the GUSTO study
| N (%) | 25(OH)D sufficiency >75.0 nmol/L N = 156 (53.4) | 25(OH)D inadequacy ≤75.0 nmol/L N = 136 (46.6) | P-value |
|---|---|---|---|
| Maternal characteristics | |||
| Ethnicity, N (%) | <0.001 | ||
| Chinese (N = 131) | 96 (61.5) | 35 (25.7) | |
| Malay (N = 115) | 39 (25.0) | 76 (55.9) | |
| Indian (N = 46) | 21 (13.5) | 25 (18.4) | |
| Parity, N (%) | 0.879 | ||
| Primiparous | 61 (39.1) | 52 (38.2) | |
| Multiparous | 95 (60.9) | 84 (61.8) | |
| Maternal education, N (%) | 0.623 | ||
| Primary and below | 8 (5.3) | 6 (4.5) | |
| Secondary/technical education | 74 (49.0) | 72 (54.1) | |
| Diploma/ University | 69 (45.7) | 55 (41.4) | |
| Age (year) | 30 ± 5 | 29 ± 5 | 0.010 |
| Pre-pregnancy BMI (kg/m2) | 22.4 ± 4.6 | 23.8 ± 5.1 | 0.019 |
| Fasting plasma glucose (mmol/L) | 4.3 ± 0.4 | 4.5 ± 0.7 | 0.055 |
| 2-h plasma glucose (mmol/L) | 6.3 ± 1.5 | 6.1 ± 1.4 | 0.250 |
| Neonatal characteristics | |||
| Sex, N (%) | 0.240 | ||
| Male (N = 161) | 91 (58.3) | 70 (51.5) | |
| Female (N = 131) | 65 (41.7) | 66 (48.5) | |
| Birth weight (kg) | 3.1 ± 0.5 | 3.2 ± 0.4 | 0.081 |
| Gestational age (wk.) | 38.7 ± 1.2 | 38.7 ± 1.2 | 0. 526 |
| Age on MRI day (d) | 10 ± 3 | 10 ± 2 | 0.166 |
| Weight on the day of MRI (kg) | 3.1 ± 0.7 | 3.2 ± 0.6 | 0.256 |
| sSAT (ml) | 74.7 ± 21.0 | 81.3 ± 22.5 | 0.010 |
| dSAT (ml) | 12.2 ± 5.2 | 14.3 ± 5.8 | 0.002 |
| IAT (ml) | 22.9 ± 8.1 | 22.9 ± 7.3 | 0.976 |
Data shown are N (%) for categorical variables or mean ± SD for continuous variables
P-values are based on between group comparisons of 25(OH)D groups using ANOVA for continuous variables and χ2-test for categorical variables among 25(OH)D groups
sSAT abdominal superficial subcutaneous adipose tissue, dSAT abdominal deep subcutaneous adipose tissue, IAT, abdominal internal adipose tissue
Inverse linear correlations were observed between maternal 25(OH)D and subcutaneous AAT volumes: r = −0.190, P = 0.001 for sSAT and r = −0.206, P < 0.001 for dSAT.
Table 2 shows AAT volumes in different quartiles (Q) of 25(OH)D (Q1: 20.0–58.2, Q2: 58.3–78.0, Q3: 78.1–95.8, Q4: 95.9–155 nmol/L). sSAT and dSAT volumes decreased with increasing quartiles of 25(OH)D. Adjusting for relevant covariates (ethnicity, sex, age on MRI day, gestational age, maternal age, maternal education, maternal pre-pregnancy BMI, and parity), multivariable regression analysis showed that each 1 nmol/L increase in 25(OH)D was associated with a reduction in sSAT (−0.14 ml; 95% CI: −0.24, −0.04 ml, P = 0.006) and dSAT (−0.04 ml; 95% CI: −0.06, −0.01 ml, P = 0.006), but was not associated with IAT.
Table 2.
Abdominal adipose tissue compartment volumes by quartiles of maternal plasma 25(OH)D levels among 292 participants in the GUSTO study
| 25(OH)D quartiles | N | Mean ± SD | P for trend | |
|---|---|---|---|---|
| Superficial subcutaneous | 1 | 73 | 81.2 ± 20.9 | 0.013 |
| adipose tissue | 2 | 75 | 80.0 ± 23.9 | |
| 3 | 71 | 76.9 ± 21.1 | ||
| 4 | 73 | 72.7 ± 20.9 | ||
| Deep subcutaneous | 1 | 73 | 13.8 ± 4.9 | 0.006 |
| adipose tissue | 2 | 75 | 14.4 ± 6.7 | |
| 3 | 71 | 13.1 ± 5.1 | ||
| 4 | 73 | 11.5 ± 5.2 | ||
| Internal adipose tissue | 1 | 73 | 22.4 ± 6.3 | 0.900 |
| 2 | 75 | 23.1 ± 8.0 | ||
| 3 | 71 | 23.7 ± 8.3 | ||
| 4 | 73 | 22.4 ± 8.1 |
Data shown are mean ± SD
25(OH)D quartile 1: 20.0–58.2, quartile 2: 58.3–78.0, quartile 3: 78.1–95.8, quartile 4: 95.9–155 nmol/L
Neonates of mothers with 25(OH)D inadequacy had greater abdominal sSAT and dSAT volumes than those of mothers with 25(OH)D sufficiency, but had similar IAT volumes. The associations remained significant after adjusting for above-mentioned covariates; differences (95% CI) were: 7.3 (2.1, 12.4) ml, P = 0.006 and 2.0 (0.6, 3.4) ml, P = 0.005 for sSAT and dSAT respectively (Table 3). Sensitivity analyses were performed to examine the robustness of the results by restricting our analyses to subgroups of neonates born >37 weeks gestational age (N = 272) and non-small for gestational age neonates born >37 weeks gestational age (N = 240) (Supplemental Table 2). The associations between 25(OH)D status and the neonatal abdominal adiposity persisted in both subgroups.
Table 3.
Mean difference in neonatal abdominal adiposity according to maternal plasma 25 (OH)D status (i.e., sufficiency vs. inadequacy) in GUSTO study
| sSAT (ml) | dSAT (ml) | IAT (ml) | |
|---|---|---|---|
| Overall MRI group (N = 292) | |||
| 25(OH)D sufficiency (>75.0 nmol/L) | Reference | Reference | Reference |
| 25(OH)D inadequacy (≤75.0 nmol/L) | 7.3 (2.1, 12.4) | 2.0 (0.6, 3.4) | 1.1 (−0.8, 2.9) |
| P = 0.006 | P = 0.005 | P = 0.268 | |
| Non-GDM group (N = 237) | |||
| 25(OH)D sufficiency (>75.0 nmol/L) | Reference | Reference | Reference |
| 25(OH)D inadequacy (≤75.0 nmol/L) | 4.8 (−0.6, 10.1) | 1.7 (0.3, 3.1) | 0.6 (−1.3, 2.4) |
| P = 0.075 | P = 0.019 | P = 0.549 | |
Associations shown are differences in mean (95% CI) of 25(OH)D inadequacy group vs the reference 25(OH)D sufficient group
P-values were determined with the use of multivariable regression models
Total sample size (N) is not always 292 or 237 due to the missing values
Models controlled for ethnicity, sex, age on MRI day, gestational week, maternal age, maternal education, maternal pre-pregnancy BMI, and parity
sSAT abdominal superficial subcutaneous adipose tissue, dSAT abdominal deep subcutaneous adipose tissue, IAT abdominal internal adipose tissue
Table 3 also demonstrates the associations of maternal 25(OH)D groups and neonatal adiposity in a subset of non-GDM mothers. The effect size for these associations declined by 34% for sSAT, 15% for dSAT and 46% for IAT. Maternal 25(OH)D insufficiency remained significantly associated with neonatal dSAT: difference (95% CI); 1.7 (0.3, 3.1) ml, P = 0.019 in this subset.
Supplemental Table 3 shows the association of maternal 25(OH)D status with neonatal adiposity after adjusting for both maternal FPG and 2HrPG for both the entire MRI cohort (N = 292) and in the non-GDM subset (N = 237). For maternal FPG in the entire MRI cohort, the associations between maternal 25(OH)D status and neonatal abdominal adiposity declined by only 19% for sSAT to 5.9 (1.0, 10.8) ml and 15% for dSAT to 1.7 (0.4, 3.0) ml compared to the unadjusted associations. However, these associations increased by 12% for sSAT, 8.3 (3.0, 13.5) ml and by 13% greater for dSAT, 2.3 (0.9, 3.7) ml after adjusting for 2HrPG.
In the non-GDM subset, the associations between maternal 25(OH)D status and neonatal adiposity persisted for dSAT; 1.6 (0.2, 2.9) ml and 1.8 (0.4, 3.2) ml after adjusting for maternal FPG and 2HrPG, respectively. For sSAT, the association persisted after adjusting for FPG: 5.4 (0.6, 10.6) ml (Supplemental Table 3).
Discussion
In this cohort study of mother-neonate pairs, inverse associations were observed between maternal mid-gestation 25(OH)D levels and neonatal abdominal subcutaneous adipose tissue compartment volumes; both sSAT and dSAT. These observed associations were present even after adjusting for maternal glycemia, both FPG and 2HrPG levels. These findings are consistent with those of previous studies in adolescents and adults, which observed inverse associations between vitamin D levels and visceral adiposity measured by computed tomography or MRI [25–28]. Maternal 25(OH)D inadequacy was also associated with greater neonatal abdominal subcutaneous adipose tissue (sSAT and dSAT) despite similar birth weight and weight on MRI day. For non-GDM mothers, the association between maternal 25(OH)D inadequacy and neonatal SAT was less pronounced but persisted for dSAT, which is more metabolically active and similar to VAT in adults. In the GUSTO cohort, lower maternal 25(OH)D status was associated with higher FPG levels [24] with a continuous gradient between maternal glycemia and excessive neonatal adiposity that extended across the range of maternal glycemia [29]. Therefore, it is expected that association between maternal 25(OH)D and neonatal adiposity would be less pronounced in non-GDM group compared to that of whole-MRI cohort.
Abdominal adiposity is known to be associated with higher risks of insulin resistance, T2DM and coronary heart disease in adult life and has been widely studied in relation to metabolic diseases [30, 31]. Abdominal adiposity is relevant especially in Asians who are at higher metabolic risk than the western population even at lower or similar BMI or waist circumference [15, 16], a conventional measure for abdominal adiposity. AAT is classically grouped into subcutaneous adipose tissue (SAT) and visceral (VAT) or internal adipose tissue. Further subdivision of SAT into sSAT and dSAT has been studied only recently. dSAT could be more relevant to metabolic parameters than sSAT and is increasingly suggested to be strongly related to increase in insulin resistance and cardio-metabolic parameters in a similar manner to VAT [32–34]. IAT in neonates includes VAT, which is the fat around the organs, intermuscular fat, paravertebral, and intra-spinal fat (Fig. 1). However, amount of VAT is minimal compared to other types of fat within abdominal region in neonates [18]. Therefore, the presence of IAT in neonates might not reflect metabolically active visceral adiposity, as it does in adults. Our findings of increased dSAT in the neonates of mothers with 25(OH)D inadequacy suggest a potential role for maternal 25(OH)D in the offspring’s susceptibility to metabolic diseases later in life.
Reported associations between maternal or umbilical cord plasma 25(OH)D status and adiposity in neonates have been mixed. Godang et al.[13] studied total fat mass (FM) measured by dual-energy X-ray absorptiometry in 202 Norwegian mother-neonate pairs. They found no association with maternal 25(OH)D at 30–32 weeks gestation but a positive association with umbilical cord plasma (UCP) 25(OH)D. Josefson et al.[14] observed a cross-sectional association between UCP 25(OH)D at 36–38 weeks gestation and FM of newborns measured by air displacement plethysmography in 61 mother-newborn pairs in Chicago [14]. The same authors found no relationship between maternal or cord blood 25(OH)D levels measured at 28 weeks and neonatal adiposity measured by skinfold thickness in a subset of subjects (N = 360) of the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [35]. In the Southampton Women’s Survey, Crozier et al.[12] found maternal 25(OH)D insufficiency at 34 weeks of gestation to be associated with lower FM in 977 neonates but greater adiposity at age 6 years. Other studies observed no association between maternal 25(OH)D and neonatal FM measured by skinfolds or bio-electrical impedance analysis [36, 37]. All these studies measured total adiposity of the offspring, not abdominal adiposity, which is known to be more strongly associated with metabolic risk. Therefore, our findings add to the limited published information on the association between maternal 25(OH)D and abdominal adiposity in Asian neonates, and our study is the first to report on these associations.
The mechanisms underlying the associations between maternal 25(OH)D levels and offspring abdominal adiposity are unclear. Current available data suggest a possible link between vitamin D level and glucose homeostasis, but the exact mechanism for role of either on the other is not well clear. Several mechanisms have been proposed including the possible effects of 25(OH)D on insulin sensitivity and glucose homeostasis. In animal studies, 25(OH)D affects circulating glucose levels by altering insulin secretion from pancreatic β-cells through vitamin D receptors in the pancreas [38, 39]. An association of 25(OH)D deficiency with β-cell dysfunction and insulin resistance has also been observed in adults. In NHANES III, a lower quartile of 25(OH)D (N = 6228) was associated with higher fasting glucose levels. The prevalence of 25(OH)-deficiency is greater in T2DM patients, and serum 25(OH)D levels are lower in newly diagnosed adult diabetics and those with impaired glucose tolerance compared to controls [40]. A cross-sectional study of healthy glucose-tolerant subjects found a significant positive correlation of 25(OH)D levels with insulin sensitivity and a negative correlation with first- and second-phase insulin response measured by hyperinsulinemic clamp [7]. These findings suggest an important role of 25(OH)D on glycemia, perhaps via endocrine functioning of the pancreatic β-cells.
In our multi-ethnic Asian cohort GUSTO, maternal vitamin D maternal 25(OH)D inadequacy was associated with higher fasting plasma glucose levels [24]. However, the associations between maternal 25(OH)D status and neonatal abdominal subcutaneous tissue persisted (though only for dSAT in neonates of the non-GDM mothers) after the adjustment for maternal FPG and 2HrPG.
Our findings may help in developing strategies to prevent vitamin D deficiency in offspring of women with (or having a high risk of) 25(OH)D insufficiency. Our observation that neonatal adiposity in very early in life is influenced by maternal 25(OH)D insufficiency independently of its effect on maternal glycemia suggests that correcting mothers’ 25(OH) deficiency may be as important as optimizing their glucose levels.
Evidence from randomized controlled trials (RCTs) is mixed on the effectiveness of vitamin D supplementation during pregnancy on offspring metabolic outcomes. Some RCTs have reported a benefit (newborns of pregnant women of the intervention groups had higher birth weight than the controls [41–44]), while others have not [45, 46]. Systematic review of the observational data concluded there was modest evidence to support a relationship between maternal 25(OH)D status and offspring birth weight [47]. The offspring’s vitamin D concentration is largely determined by maternal vitamin D status, and maintaining maternal vitamin D status through supplementation might therefore benefit neonatal metabolic outcomes such as adiposity. Further observational and maternal vitamin D supplementation studies are needed; for example, childhood adiposity assessment is currently underway in the MAVIDOS randomized control trial [48].
Our study has several strengths. To our knowledge, it is the first study to examine the association between maternal 25(OH)D status and abdominal adipose tissue distribution in neonates using MRI. MRI is the only possible method to quantify AAT compartment volumes without radiation. We used a more robust semi-automated approach for segmentation of AAT; initial auto-segmentation by in-house segmentation algorithm followed by manual optimization performed by trained image analysts. Using MRI to measure AAT has enabled us to demonstrate the impact of maternal 25(OH)D levels on neonatal abdominal adiposity. This might not have been possible using conventional measures, such as birth weight or even total body fat. A recent report from a randomized controlled trial by Wagner and Hollis did not observe a difference in birth weight among neonates whose mothers received different doses of vitamin D supplementation during pregnancy, even though a sufficient vitamin D level (>80 nmol/L) was achieved in the group receiving 4000 IU/day (vs 400 and 2000 IU/day) [49].
In addition, our sample size (N = 292) is larger than those of the few previous studies using MRI to measure abdominal adiposity [50, 51]. Another strength is the use of MS/MS, the gold standard for measuring 25(OH)D. The competitive binding assays commonly used for measuring 25(OH)D systematically underestimate vitamin D levels, owing to differences in antibody affinity.
One limitation of our study is our single measurement of maternal 25(OH)D. 25(OH)D is known to increase throughout pregnancy to compensate the fetus’s increasing demand for growth and development [52]. If the association between 25(OH)D and neonatal abdominal adiposity is more pronounced during the third trimester, we may have underestimated the magnitude of this association. Another limitation of our study is the absence of neonatal or cord blood 25(OH)D measurements, which would allow us to verify the levels transferred to the neonate. However, previous studies have found maternal 25(OH)D status during pregnancy to be strongly correlated with both cord blood [56, 57] and later offspring 25(OH)D status [11, 58]. Nor did we measure vitamin D binding protein (DBP) and therefore cannot determine whether 25(OH) levels in sufficient and inadequate groups were due to true differences in free 25(OH)D levels or variations in DBP levels. Another potential source of bias is that mothers of non-participating neonates had higher mean maternal 25(OH)D level than mothers of participants, despite a similar mean birth weight between neonates of these two groups. It is possible that inclusion of non-participating neonates might have changed the observed associations between maternal 25(OH)D and neonatal abdominal adiposity. In addition, as in any other observational study, residual confounding may have affected our results, despite our adjustment for measured potential confounders.
Our study supports an extended role of 25(OH)D in fetal development and the offspring’s body composition. Our findings provide novel insight into the associations between maternal vitamin D and neonatal adiposity, suggesting that these associations are largely independent of maternal glucose levels. Observed greater abdominal adiposity in neonates born to in mothers with 25(OH)D inadequacy may place the neonates at higher risk of cardio-metabolic diseases later in life. Since increased central fat deposition is a potentially modifiable risk factor for T2DM and CVD, our findings, if replicated in both Asian and western population, may have important public health implications. Future randomized interventional trials with long-term follow-up will help establish if the observed associations are causal and to assess the value of vitamin D supplementation during pregnancy.
Electronic supplementary material
Acknowledgements
All authors thank the GUSTO study group, Department of Diagnostic and Interventional Imaging, KKH, Department of Diagnostic Imaging, NUH. The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit F.P. Broekman, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Chen, Chen Ling Wei, Yin Bun Cheung, Amutha Chinnadurai, Chai Kiat Chng, Shang Chee Chong, Mei Chien Chua, Doris Fok, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Joanna D. Holbrook, Chin-Ying Hsu, Neerja Karnani, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Ngee Lek, Sok Bee Lim, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Krishnamoorthy Niduvaje, Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Su, Kok Hian Tan, Oon Hoe Teoh, Hugo P S van Bever, Inez Bik Yun Wong, P. C. Wong, and George Seow Heong Yeo.
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; Additional funding of the present study was provided by the Singapore Institute for Clinical Sciences, A*STAR and Nestec. K.M.G. is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as an NIHR Senior Investigator (NF-SI-0515-10042) and through the NIHR Southampton Biomedical Research Centre) and the European Union’s Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977. Study sponsors were not involved in the design of the study, statistical analysis and interpretation of results.
Author contributions
M.T.T. performed statistical analysis and wrote the manuscript. M.T.T. and M.V.F. contributed to MR image acquisition, analysis and interpretation of images. I.M.A. contributed to statistical analysis. J.K. contributed to MR image acquisition and V.S.R. was responsible for neonatal data acquisition. M.F.C. and P.L.Q. were responsible for 25(OH)D data. M.F.C., K.M.G., M.S.K., C.J.H., and Y.S.L. contributed to critical revision of the manuscript for important intellectual content. S.S.M., F.Y., Y.S.L., K.M.G., P.D.G., and Y.S.C. designed and led GUSTO study. M.V.F. and Y.S.L. have primary responsibility for the final content of the manuscript. M.T.T. and Y.S.L. are joint corresponding authors. All authors have read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Mya Thway Tint, Phone: +65-660 11947, Email: obgmtt@nus.edu.sg.
Yung Seng Lee, Phone: +65-6772 4420, Email: paeleeys@nus.edu.sg.
Electronic supplementary material
The online version of this article (10.1038/s41366-018-0032-2) contains supplementary material, which is available to authorized users.
References
- 1.WHO. http://www.who.int/end-childhood-obesity/facts/en/
- 2.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 3.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19:43–55. doi: 10.1385/ENDO:19:1:43. [DOI] [PubMed] [Google Scholar]
- 4.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 5.Martini LA, Wood RJ. Vitamin D status and the metabolic syndrome. Nutr Rev. 2006;64:479–86. doi: 10.1111/j.1753-4887.2006.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 8.Roth CL, Elfers C, Kratz M, Hoofnagle AN. Vitamin D deficiency in obese children and its relationship to insulin resistance and adipokines. J Obes. 2011;2011:495101. doi: 10.1155/2011/495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–4. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 11.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–7. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 12.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96:57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godang K, Froslie KF, Henriksen T, Qvigstad E, Bollerslev J. Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. Eur J Endocrinol. 2014;170:609–17. doi: 10.1530/EJE-13-0842. [DOI] [PubMed] [Google Scholar]
- 14.Josefson JL, Feinglass J, Rademaker AW, Metzger BE, Zeiss DM, Price HE, et al. Maternal obesity and vitamin D sufficiency are associated with cord blood vitamin D insufficiency. J Clin Endocrinol Metab. 2013;98:114–9. doi: 10.1210/jc.2012-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair M, Prabhakaran D. Why do South Asians have high risk for CAD? Glob Heart. 2012;7:307–14. doi: 10.1016/j.gheart.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Gordon-Larsen P, Adair LS, Meigs JB, Mayer-Davis E, Herring A, Yan SK, et al. Discordant risk: overweight and cardiometabolic risk in Chinese adults. Obesity. 2013;21:E166–74. doi: 10.1002/oby.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 18.Tint MT, Fortier MV, Godfrey KM, Shuter B, Kapur J, Rajadurai VS, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: Growing Up in Singapore Toward Healthy Outcomes birth cohort study. Am J Clin Nutr. 2016;103:1311–7. doi: 10.3945/ajcn.115.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51:1683–90. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med: J Br Diabet Assoc. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Ong YL, Quah PL, Tint MT, Aris IM, Chen LW, van Dam RM, et al. The association of maternal vitamin D status with infant birth outcomes, postnatal growth and adiposity in the first 2 years of life in a multi-ethnic Asian population: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Br J Nutr. 2016;116:621–31. doi: 10.1017/S0007114516000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Fakhri N, McDevitt H, Shaikh MG, Halsey C, Ahmed SF. Vitamin D and its effects on glucose homeostasis, cardiovascular function and immune function. Horm Res Paediatr. 2014;81:363–78. doi: 10.1159/000357731. [DOI] [PubMed] [Google Scholar]
- 24.Loy SL, Lek N, Yap F, Soh SE, Padmapriya N, Tan KH, et al. Association of maternal Vitamin D status with glucose tolerance and caesarean section in a multi-ethnic Asian cohort: The Growing Up in Singapore Towards Healthy Outcomes Study. PLoS ONE. 2015;10:e0142239. doi: 10.1371/journal.pone.0142239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulistyoningrum DC, Green TJ, Lear SA, Devlin AM. Ethnic-specific differences in vitamin D status is associated with adiposity. PLoS ONE. 2012;7:e43159. doi: 10.1371/journal.pone.0043159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannemann A, Thuesen BH, Friedrich N, Volzke H, Steveling A, Ittermann T, et al. Adiposity measures and vitamin D concentrations in Northeast Germany and Denmark. Nutr Metab. 2015;12:24. doi: 10.1186/s12986-015-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–11. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, et al. Effect of maternal glycemia on neonatal adiposity in a multiethnic Asian birth cohort. J Clin Endocrinol Metab. 2014;99:240–7. doi: 10.1210/jc.2013-2738. [DOI] [PubMed] [Google Scholar]
- 30.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab: Clin Exp. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 31.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 33.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–7. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–9. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 35.Josefson JL, Reisetter A, Scholtens DM, Price HE, Metzger BE, Langman CB. Maternal BMI associations with maternal and cord blood vitamin D levels in a North American Subset of Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Participants. PLoS ONE. 2016;11:e0150221. doi: 10.1371/journal.pone.0150221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–52. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Investig. 1984;73:759–66. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 40.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol. 2006;65:593–7. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 41.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maghbooli Z, Hossein-Nezhad A, Shafaei AR, Karimi F, Madani FS, Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy Childbirth. 2007;7:1. doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–8. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 44.Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol. 2015;83:536–41. doi: 10.1111/cen.12751. [DOI] [PubMed] [Google Scholar]
- 45.Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab. 2014;99:2448–55. doi: 10.1210/jc.2013-3491. [DOI] [PubMed] [Google Scholar]
- 46.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol. 2009;70:685–90. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 47.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18:1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double blind, randomized clinical trial of safety and effectiveness. J Bone Mineral Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatr Res. 2009;65:584–7. doi: 10.1203/PDR.0b013e31819d98be. [DOI] [PubMed] [Google Scholar]
- 51.Logan KM, Emsley RJ, Jeffries S, Andrzejewska I, Hyde MJ, Gale C, et al. Development of early adiposity in infants of mothers with gestational diabetes mellitus. Diabetes Care. 2016;39:1045–51. doi: 10.2337/dc16-0030. [DOI] [PubMed] [Google Scholar]
- 52.Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HM, et al. Tracking of 25-hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. Am J Clin Nutr. 2015;102:1081–7. doi: 10.3945/ajcn.115.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


