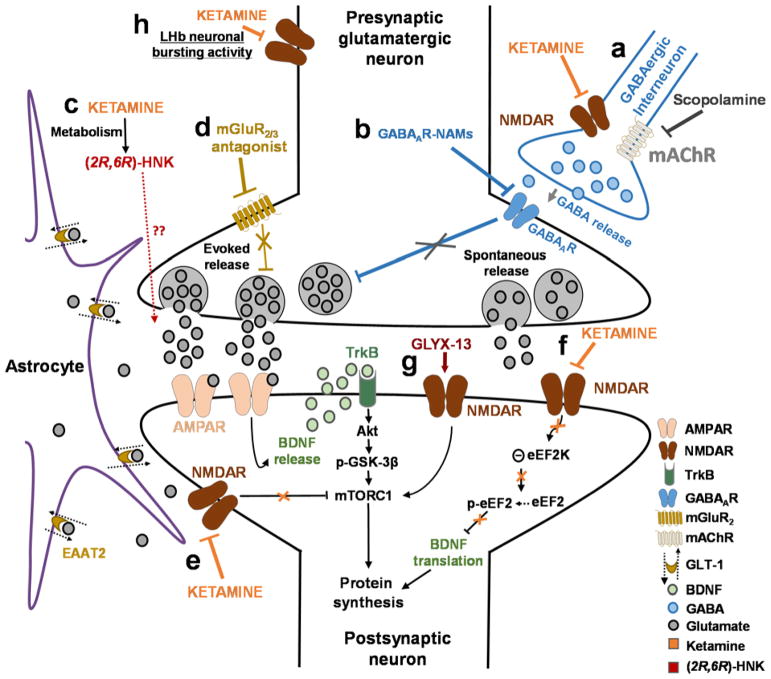
Figure 1. Proposed mechanisms of action of ketamine and other putative rapid-acting antidepressant.
(A) Disinhibition hypothesis: ketamine or scopolamine selectively block N-methyl-D-aspartate receptors (NMDARs) or muscarinic acetylcholine receptors (mAChRs), respectively, expressed on GABAergic inhibitory interneurons, causing a decrease in interneuron activity, which leads to a disinhibition of pyramidal neurons and enhanced glutamatergic firing. (B) Negative modulators of GABAA receptors (GABAAR-NAMs) directly act to reduce pyramidal neuron inhibition. Evoked released glutamate binds to and activates post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR). (C) Role of ketamine metabolites: Ketamine exerts NMDAR inhibition-independent antidepressant actions via the action of its metabolite, (2R,6R)-hydroxynorketamine (HNK), which acts to promote glutamate release (unpublished data) and AMPAR-mediated synaptic potentiation. (D) Antagonists of the group II metabotropic glutamate receptors (mGluR2/3) disinhibit the tonic blockade of presynaptic glutamate release, thus enhancing synaptic glutamatergic neurotransmission and thus inducing AMPAR activation. AMPAR activation results in enhanced brain-derived neurotrophic factor (BDNF) release, activation of the tropomyosin receptor kinase B (TrkB) receptor, and a subsequent promotion of protein synthesis via the activation of the mechanistic target of rapamycin complex 1 (mTORC1 complex). (E) Inhibition of extra-synaptic NMDARs: Ketamine selectively blocks extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the glutamate transporter 1 (EAAT2) located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs de-suppresses mechanistic target of rapamycin complex 1 (mTORC1) function, which in turn induces protein synthesis. (F) Blockade of spontaneous NMDAR activation: Ketamine blocks NMDAR-mediated spontaneous neurotransmission (miniature excitatory postsynaptic currents – mEPSC), which results in the inhibition of the eukaryotic elongation factor 2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently leads to an enhancement of BDNF translation and ultimately protein synthesis. (G) GLYX-13-induced partial activation of NMDARs: Activation of the NMDARs is hypothesized to activate mTORC1 and thus to induce protein synthesis. (H) Inhibition of NMDAR-dependent burst firing activity of lateral habenula (LHb) neurons: ketamine is proposed to decrease excessive NMDAR-dependent burst firing of LHb neurons linked to depressive symptomatology. All hypotheses propose sustained changes in synaptic plasticity, leading to strengthening of excitatory synapses, being necessary for antidepressant responses. Abbreviations: EAAT2, excitatory amino acid transporter 2; GABA, gamma aminobutyric acid; GSK, glycogen synthase kinase