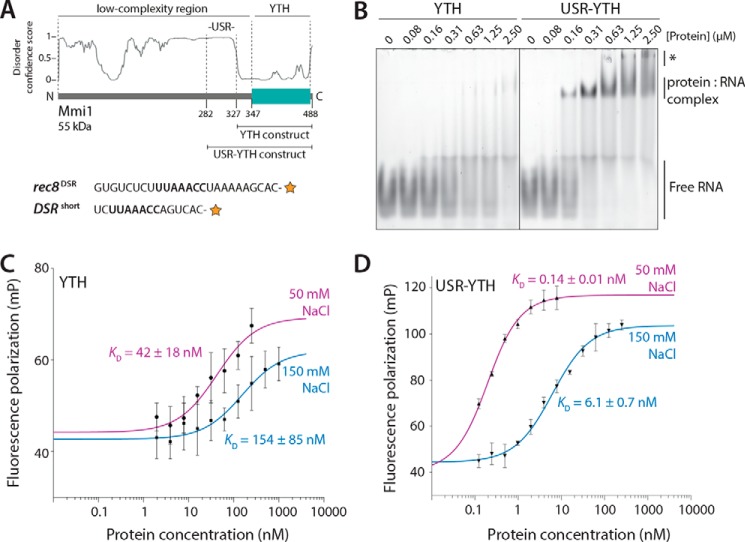
Figure 1.
The USR increases the affinity of interaction of the Mmi1 YTH domain with RNA. A, top, schematic diagram of Mmi1 domain architecture, with the YTH and USR–YTH constructs indicated. The disorder prediction from DISOPRED3 is shown. Bottom, RNAs used for EMSAs (rec8DSR) and fluorescence polarization (DSRshort). The orange star represents the 3′-fluorescein label, and the UNAAAC motif is in bold. B, USR–YTH construct binds RNA more stably than the YTH domain alone. A fluorescently-labeled rec8DSR RNA containing the Mmi1 DSR motif was analyzed by EMSA after incubation with purified proteins at the indicated concentrations. Binding was analyzed by native PAGE. Free RNA, shifted protein–RNA complex, and higher-order supershifted complexes (asterisk) are indicated. C and D, fluorescence polarization assays of YTH (C) and USR–YTH (D) binding to DSRshort RNA (used at 0.1 nm). Calculated KD values are indicated, and error bars are the standard deviation of five biological replicates (each with three technical replicates).