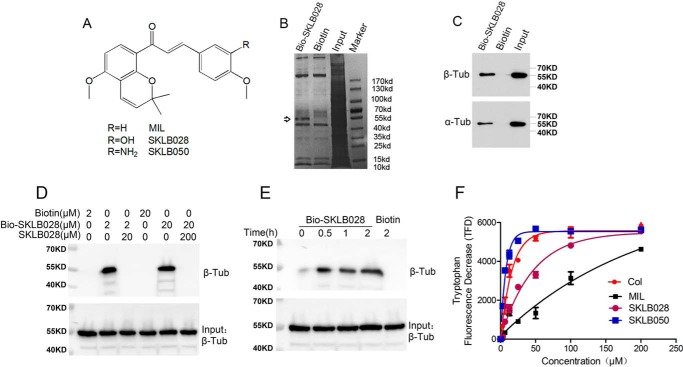
Figure 1.
MIL directly targets β-tubulin. A, chemical structures of MIL. B, HepG2 cell lysates were incubated with Bio-SKLB028 or biotin, followed by pulldown with streptavidin–agarose. The precipitates were resolved by SDS-PAGE, and the gel was stained with Coomassie staining. C, HepG2 cell lysates were incubated with Bio-SKLB028 or biotin, followed by pulldown with streptavidin–agarose. The precipitates were detected by Western blotting by α- and β-tubulin. D, porcine brain tubulin (1 μm) were preincubated with or without indicated concentrations of SKLB028 for 2 h before incubation with lower concentration of Bio-SKLB028 for another 2 h, and different concentrations of biotin were incubated with tubulin for 2 h as control groups. Then all the samples were pulled down by streptavidin–agarose beads, and the eluted samples were bolted for β-tubulin. Also, total protein for each sample was detected by Western blotting for β-tubulin as a loading control. E, porcine brain tubulin (1 μm) was incubated with Bio-SKLB028 for different times and then pulled down by a streptavidin–agarose beads, and the eluted samples were bolted for β-tubulin. Also, total protein for each sample was detected by Western blotting for β-tubulin as a loading control. F, tryptophan-based binding assay to detect the Kd value of indicated compounds binding to tubulin. The indicated compounds at different concentrations (3.125, 6.25, 12.5, 25, 50, 100, and 200 μm) were incubated with tubulin for 30 min and then monitored at 295 nm (excitation) and 335 nm (emission) using Biotech Gen5 spectrophotometer. The dissociation constants were calculated from fitting curves of decreasing fluorescence using the GraphPad Prism software. Col, colchicine; Tub, tubulin; Col, colchicine.